Comparison of two fluid warming devices for maintaining body core temperature during living donor liver transplantation: Level 1 H-1000 vs. Fluid Management System 2000
Article information
Abstract
Background
Rapid fluid warming has been a cardinal measure to maintain normothermia during fluid resuscitation of hypovolemic patients. A previous laboratory simulation study with different fluid infusion rates showed that a fluid warmer using magnetic induction is superior to a warmer using countercurrent heat exchange. We tested whether the simulation-based result is translated into the clinical liver transplantation.
Methods
Two hundred twenty recipients who underwent living donor liver transplantation between April 2009 and October 2011 were initially screened. Seventeen recipients given a magnetic induction warmer (FMS2000) were matched 1 : 1 with those given a countercurrent heat exchange warmer (Level-1 H-1000) based on propensity score. Matched variables included age, gender, body mass index, model for end-stage liver disease score, graft size and time under anesthesia. Core temperatures were taken at predetermined time points.
Results
Level-1 and FMS groups had comparable core temperature throughout the surgery from skin incision, the beginning/end of the anhepatic phase to skin closure. (P = 0.165, repeated measures ANOVA). The degree of core temperature changes within the dissection, anhepatic and postreperfusion phase were also comparable between the two groups. The minimum intraoperative core temperature was also comparable (Level 1, 35.6℃ vs. FMS, 35.4℃, P = 0.122).
Conclusions
A countercurrent heat exchange warmer and magnetic induction warmer displayed comparable function regarding the maintenance of core temperature and prevention of hypothermia during living donor liver transplantation. The applicability of the two devices in liver transplantation needs to be evaluated in various populations and clinical settings.
Introduction
Liver transplantation, which is now considered the standard treatment option for end-stage liver diseases, is accompanied by substantial blood loss due to both underlying coagulopathy originating from liver diseases and progressive coagulopathy during transplantation due to the extraction of liver. Accordingly, rapid infusion of large volume of fluids and blood products is common during liver transplantation and can significantly decrease body core temperature (BCT) and consequently lead to hypothermia. As hypothermia is known to result in multiple adverse effects including coagulopathy and cardiac arrhythmia [1,2], rapid fluid warming has represented an important part of anesthetic management during liver transplantation [3,4,5].
Although many fluid warming devices have been introduced for intraoperative use, most of them proved to be insufficient for warming large amount of cool fluids to physiologic temperature in a short period. Meanwhile, fluid warmers using either countercurrent heat exchange or magnetic induction have demonstrated superiority over other types of warmers using dry heat or water bath technology during rapid fluid infusion [6,7,8]. A laboratory study suggested that magnetic induction was superior to countercurrent heat exchange based on simulations with various infusion rates [9]. However, sufficient clinical data has been lacking in this context and it is unclear whether the simulation-based results could be translated into a clinical setting. Moreover, thermal dynamics during liver transplantation is different from laboratory simulations, as well as other surgeries. Thus, we aimed to compare the rapid warming performances of the two fluid warmers in the clinical liver transplantation setting by look at body core temperature during procedure and hemodynamic parameters.
Materials and Methods
The Institutional Review Board approved a case-control study and waived the requirement for written informed consent. Medical records of 220 recipients who underwent elective adult-to-adult living donor liver transplantation between April 2009 and October 2011 were initially screened. Exclusion criteria included autonomic neuropathy, thyroid dysfunction, use of a heated-humidifier during transplantation [5], nonuse of a pulmonary arterial catheter, and induced hypothermia. Sample size (n = 17 for each group) was determined based on our previous study which compared the warming function of an active vs. passive airway humidifier during living donor liver transplantation [5]. Seventeen randomly selected recipients who were given a countercurrent heat exchange fluid warmer (Smiths Medical, Level 1H-1000, Rockland, MA) were matched 1 : 1 with recipients who were given a magnetic induction fluid warmer (Belmont Instrument, Fluid Management system [FMS] 2000, Billerica, MA) with the following factors contributing to the propensity score [10]: age, gender, body mass index, the model for end-stage-liver disease (MELD) score, graft-to-recipient weight ratio and time under anesthesia. The caliper for inclusion was defined as 0.2 standard deviation of the logit of the propensity score [11]. BCT and cardiac output measurements from pulmonary arterial catheter were recorded on an hourly basis and additionally at the time of skin incision (baseline), and the beginning and end of the anhepatic phase. Preoperative core temperature was not considered as a baseline because it was measured via the tympanic membrane [12].
Recipients underwent the standardized anesthetic management. Anesthesia was induced with 5 mg/kg thiopental sodium and sevoflurane, and maintained with isoflurane with the bispectral index maintained in the 40-60 range. Neuromuscular blockade was achieved using a continuous infusion of vecuronium at a rate of 0.6-0.8 µg/kg/min. Mechanical ventilation was delivered with the mixture of medical air and oxygen at a flow rate of 2 L/min, and was controlled to obtain a tidal volume of 8-10 ml/kg and to maintain normocapnea. Post end-expiratory pressure was selectively applied when the ratio of arterial oxygen partial pressure and fraction of inspired oxygen was < 200 mmHg. A large-bore central venous catheter of 9 Fr was placed in combination with a pulmonary arterial catheter (Swan-GanzCCOmboV, Edward Lifesciences, LLC, Irvine, CA). The ambient temperature was thermostatically controlled at 26℃ at anesthetic induction and 24℃ thereafter. A circulating water mattress (Blanketrol II, Clininnati Sub-Zero Products, Inc, Ohio) set at 40℃ was placed over the operating bed. A passive humidifier was placed for airway warming. Patients' arms were wrapped with vinyl covers to protect them from getting wet. Administered fluids and transfused blood products were warmed using one of two tested devices (Level 1 or FMS) from the end of anesthetic induction with the exception of 5% albumin, dextrose solutions, cryoprecipitates and platelet concentrates. The use of Level 1 or FMS was decided at the discretion of attending anesthesiologists. The fluid warmers were directly connected to the largest bore of the central venous catheter. Forced-air warming was not used due to the risk of airborne contamination and consequently risking infection of the surgical site [13,14]. Procured grafts were perfused with histidine-tryptophan-ketoglutarate solution at around 5℃. After graft reperfusion, the surgical field was irrigated and washed using warm saline.
Data were analyzed using SPSS 19.0. Continuous variables were described as median with interquartile (25 percentile, 75 percentile) and analyzed using Mann-Whitney U test. Data on BCT, fluids management, and hemodynamics were described according to the operative phases which were defined as follows: the dissection phase, from skin incision to portal triad clamping; the anhepatic phase, from portal triad clamping to portal vein unclamping (graft reperfusion); and the reperfusion phase, from portal vein unclamping to skin closure. Repeatedly measured BCT values were further analyzed using repeated measures ANOVA test for evaluating overall values during surgery. Categorical variables were described as number (%) and analyzed using chi-square test. A P value of < 0.05 was considered statistically significant.
Results
Demographic data of 34 recipients were described in Table 1. As expected, the matched variables, including body mass index, the MELD score, graft size (graft-to-recipient weight ratio), and time under anesthesia, were comparable between Level 1 and FMS group. Along with the matched variables, the two groups were also comparable regarding the preoperative BCT, baseline BCT, duration of the dissection/anhepatic/postreperfusion phase. One recipient in FMS group was given 5 mmHg positive end-expiratory pressure. BCT dropped significantly during the anhepatic phase and gradually recovered after reperfusion (Fig. 1). BCTs were not significantly different at the start (Level 1 group 36.0℃ vs. FMS group 35.9℃ , P = 0.522) and end of the anhepatic phase (Level 1 group 35.9℃ vs. FMS group 35.4℃, P = 0.085). The minimum BCT during transplantation was also comparable between both groups (Level 1 group 35.6℃ vs. FMS group 35.4℃, P = 0.122). Repeated measures ANOVA showed that overall intraoperative BCTs of the two groups were comparable (P = 0.255). The changes in BCT within the respective operative phases were measured (Table 1). The degree of BCT drop during the anhepatic phase was comparable (Level 1 group -0.4℃ vs. FMS group -0.3℃, P = 0.150). The degree of BCT rise during the postreperfusion phase was not significantly different with a marginality (Level 1 group 0.3℃ vs. FMS group 0.6℃, P = 0.067). Detailed data on the amount of infused fluids and blood products during each transplant phase are described in Table 2, showing comparable infused volumes in the two groups. As shown in Table 3, hemodynamic parameters, including cardiac output and mean arterial pressure, were also comparable between the two groups. The maximum dose of two major vasoactive drugs which were frequently used during liver transplantation was also comparable.
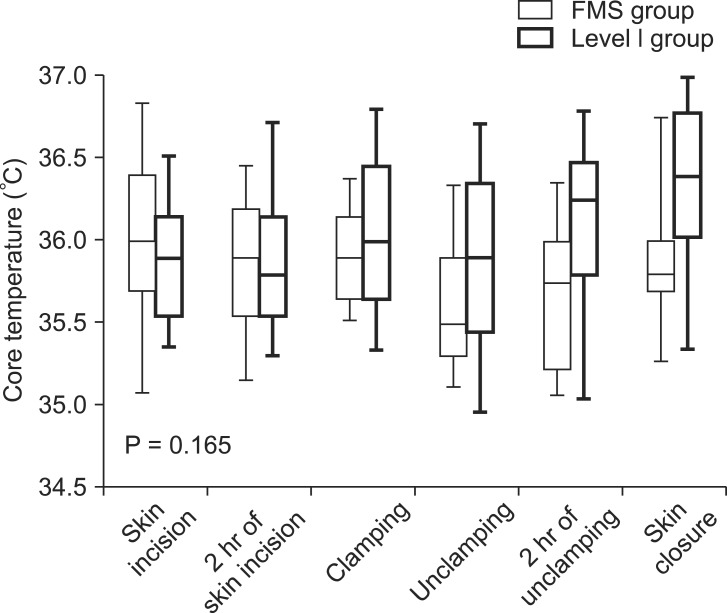
Changes in body core temperature during transplantation of recipient given FMS or Level 1 for fluids warming (P value was calculated by means of repeated measures ANOVA).
Discussion
Various commercially available fluid warming devices have been introduced and are being used in practice. Among them, Level 1 has shown superiority over other fluid warmers because it has been shown to deliver normothermic fluids irrespective of the fluid infusion rate. In a simulated evaluation, Level 1 effectively warmed fluids at low- (6.5 ml/min), moderate- (13.5-25 ml/min), and high (infusion with the roller clamp wide open at gravity flow or infusion under pressure of 300 mmHg) infusion rates [7]. Meanwhile, a previous laboratory evaluation reported that a newer device named FMS 2000 warmed fluids more efficiently compared to Level 1 at a high flow rate [9]. Thus, we conducted a clinical study to test the warming function of Level 1 and FMS 2000 during liver transplantation in which massive blood loss is expected and high rate of infusion is anticipated.
Level 1 circulates water at 42℃ through the aluminum tube-in-tube heat exchanger in the disposable set (DI-100 in the present study). The circulating water bath and the infusate flow against each other, or counter-current, and thus maximize heat transfer. In contrast, FMS 2000 warms fluids using magnetic induction. The disposable set has a stainless-steel heat exchanger in a ring type, which is inserted on the machine over the electromagnet. Electromagnetic power is controlled according to the infusate inflow/outflow temperatures measured by onboard thermistors.
In the present study, the two devices showed comparable function regarding the maintenance of intraoperative BCT and prevention of hypothermia. This finding was contrary to a previous laboratory study performed by Comunale ME who reported only FMS 2000 and not Level 1 could deliver normothermic fluids at high fluid rate (> 500 ml/min) [9]. The conflicting results could be explained by the gap between an experimental setting and clinical practice. First, infused fluids in the present study including packed red blood cells were supposed to be greater than 8℃ prior to infusion due to the ambient temperature set at 24℃ in comparison with the simulation in which inflow temperature of red blood cells was set at 8℃. Such temperature difference might be considerable and not relevant to the clinical setting. Second, the median infusion rate was around 1,000 ml/hr (17 ml/min) in the present study, while extremely rapid infusion was done in the simulation test. Our data clearly indicated that warming functions of different fluid warmers can vary by surgeries or the clinical settings. For example, slow to moderate infusion rates were applied during most of intraoperative period and relatively slow infusion rate compared to the simulation might lower the advantage of FMS 2000 regarding rapid fluid warming. In contrast, a higher set point of Level 1 (42℃) might have a thermal advantage at slow infusion rates compared to FMS 2000 (40℃). This premise was supported by the significant difference in BCT at the end of transplantation. The difference between the two groups seemed to originate from the postreperfusion phase at which the grafts start to function and coagulation profiles improve, which is associated with a lower risk of further major blood loss (Fig. 1).
Aside from warming function, the two devices are different in ease of use and safety as well as cost. FMS 2000 supplies multiple user-friendly conditions and has a high level of safety. Physicians can control the flow rate from 2.5-750 ml/min at the touch of a screen while watching real time infusate outflow temperature, line pressure and total infused volume on the same screen. Infusion automatically stops at an inappropriately high infusion pressure which is related to inappropriate status of the catheter or extension lines. Most importantly, two ultrasonic air detectors and an automatic air purge eliminate the risk of inadvertent air embolism [9]. The automatic air eliminator incorporated into Level 1 was reported to be insufficient to eliminate a 10 ml bolus of air injected into the proximal site of the heat exchanger and thus extreme caution in this context is necessary [9]. In contrast, the FMS 2000 reservoir is designed to continuously infuse large volume of fluids, making it hard to estimate the exact amount of each infused fluids and blood products, including red blood cells and fresh frozen plasma. Adequate replacement of such blood products are important because optimal blood hemoglobin concentration and hemostatic status are required to supply sufficient oxygen to the graft, to escape major bleeding, and to prevent vascular thrombosis in portal and hepatic vasculature. The high cost is an intrinsic disadvantage of the FMS 2000. In short, Level 1 and FMS have their own advantages and disadvantages which might manifest differently according to the users' experience.
The following were limitations of the present study. First, this study was not randomized. Although matched variables included major factors contributing to BCT during transplantation, there was a possibility of hidden biases from unmeasured or unmeasurable variables. Second, the MELD score, amount of blood loss, and fluids infusion rates were relatively low. Thus, data of the present study cannot be extrapolated into liver transplantation for recipients with more advanced liver diseases, which might indicate a greater amount of blood loss and infusion of fluids as well as poorer thermal homeostasis. Third, transplantations in the study were performed by expert liver transplant surgeons. The amount of blood loss and required fluids and blood products during liver transplantation are known to be closely associated with the attending surgeons' experience. Thus, the results of the study may vary if transplantation is performed by unexperienced surgeons. Forth, the difference in BCT between the two groups seemed to increase after graft reperfusion. During the reperfusion phase, the liver graft initiate its metabolic function including hepatic heat production [15]. Thus, BCT changes after graft reperfusion might be affected by the extent of hepatic ischemia reperfusion injury and initial graft quality/function [16].
Because there have been lack of clinical studies evaluating the fluid warming function of Level 1 and FMS 2000, we compared the two devices regarding their capacity to maintain intraoperative core temperature and circulating volume. It was found that the two devices were comparable in regards to maintaining intraoperative normothermia, preventing core hypothermia and maintaining hemodynamics during living donor liver transplantation at which the fluid infusion rate was mostly in the moderate level range. Thus, it might be reasonable to use one of the two devices during living donor liver transplantation according to physicians' preferences and experiences.