 |
 |
| Korean J Anesthesiol > Volume 76(5); 2023 > Article |
|
Abstract
Background
Methods
Results
NOTES
Funding
This work was supported by the Scientific Research Projects Coordination Unit of Suleyman Demirel University (Project number TSG-2020-8134).
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author Contributions
Eyy├╝p Sabri ├¢zden (Conceptualization; Data curation; Investigation; Methodology; Project administration; Supervision; Validation; Visualization; Writing ŌĆō original draft; Writing ŌĆō review & editing)
Halil A┼¤c─▒ (Conceptualization; Data curation; Funding acquisition; Investigation; Methodology; Project administration; Supervision; Validation; Visualization; Writing ŌĆō original draft; Writing ŌĆō review & editing)
Halil ─░brahim B├╝y├╝kbayram (Data curation; Formal analysis; Methodology; Resources; Software; Supervision; Validation; Visualization; Writing ŌĆō original draft; Writing ŌĆō review & editing)
Mehmet Abdulkadir Sev├╝k (Data curation; Investigation; Project administration; Validation; Visualization; Writing ŌĆō original draft; Writing ŌĆō review & editing)
Orhan Berk ─░meci (Data curation; Investigation; Project administration; Validation; Visualization; Writing ŌĆō original draft; Writing ŌĆō review & editing)
Hatice K├╝bra Do─¤an (Formal analysis; Methodology; Software; Writing ŌĆō original draft; Writing ŌĆō review & editing)
├¢zlem ├¢zmen (Conceptualization; Data curation; Formal analysis; Methodology; Supervision; Validation; Visualization; Writing ŌĆō original draft; Writing ŌĆō review & editing)
Fig.┬Ā1.
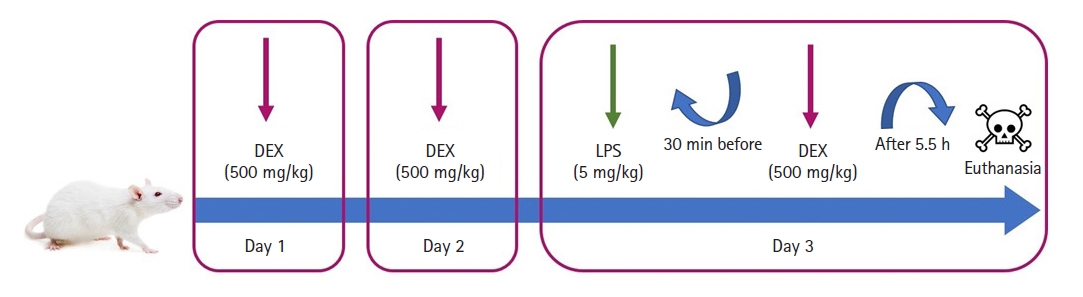
Fig.┬Ā2.

Fig.┬Ā3.
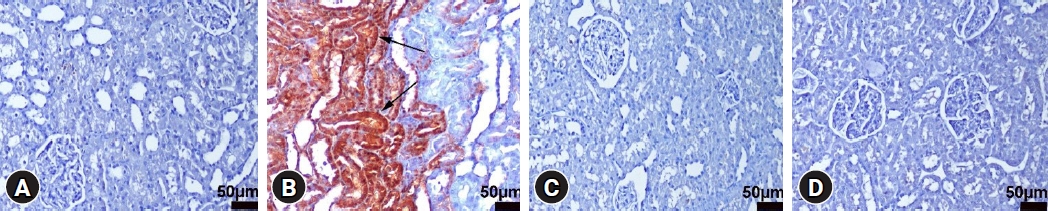
Fig.┬Ā4.
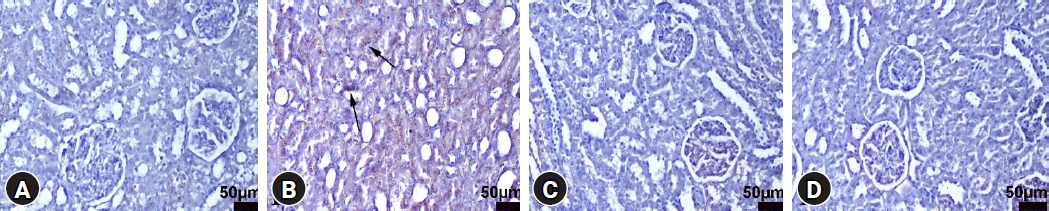
Fig.┬Ā5.
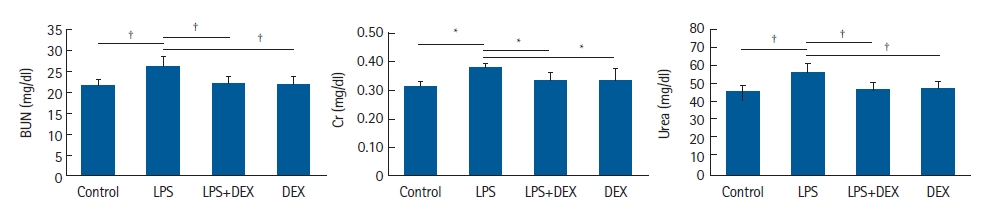
Fig.┬Ā6.
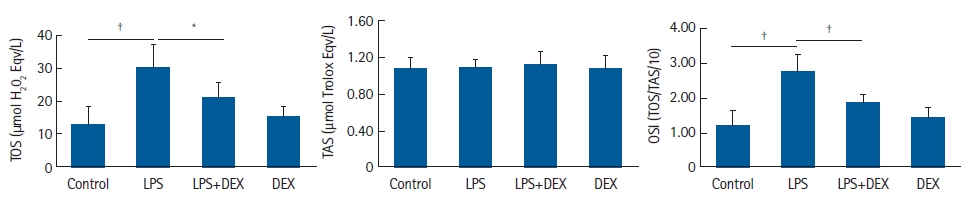
Fig.┬Ā7.










