 |
 |
| Korean J Anesthesiol > Volume 77(1); 2024 > Article |
|
Abstract
NOTES
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author Contributions
Seok Kyeong Oh (Conceptualization; Validation; Visualization; Writing ŌĆō original draft; Writing ŌĆō review & editing)
Young Ju Won (Validation; Visualization; Writing ŌĆō original draft; Writing ŌĆō review & editing)
Byung Gun Lim (Conceptualization; Supervision; Visualization; Writing ŌĆō original draft; Writing ŌĆō review & editing)
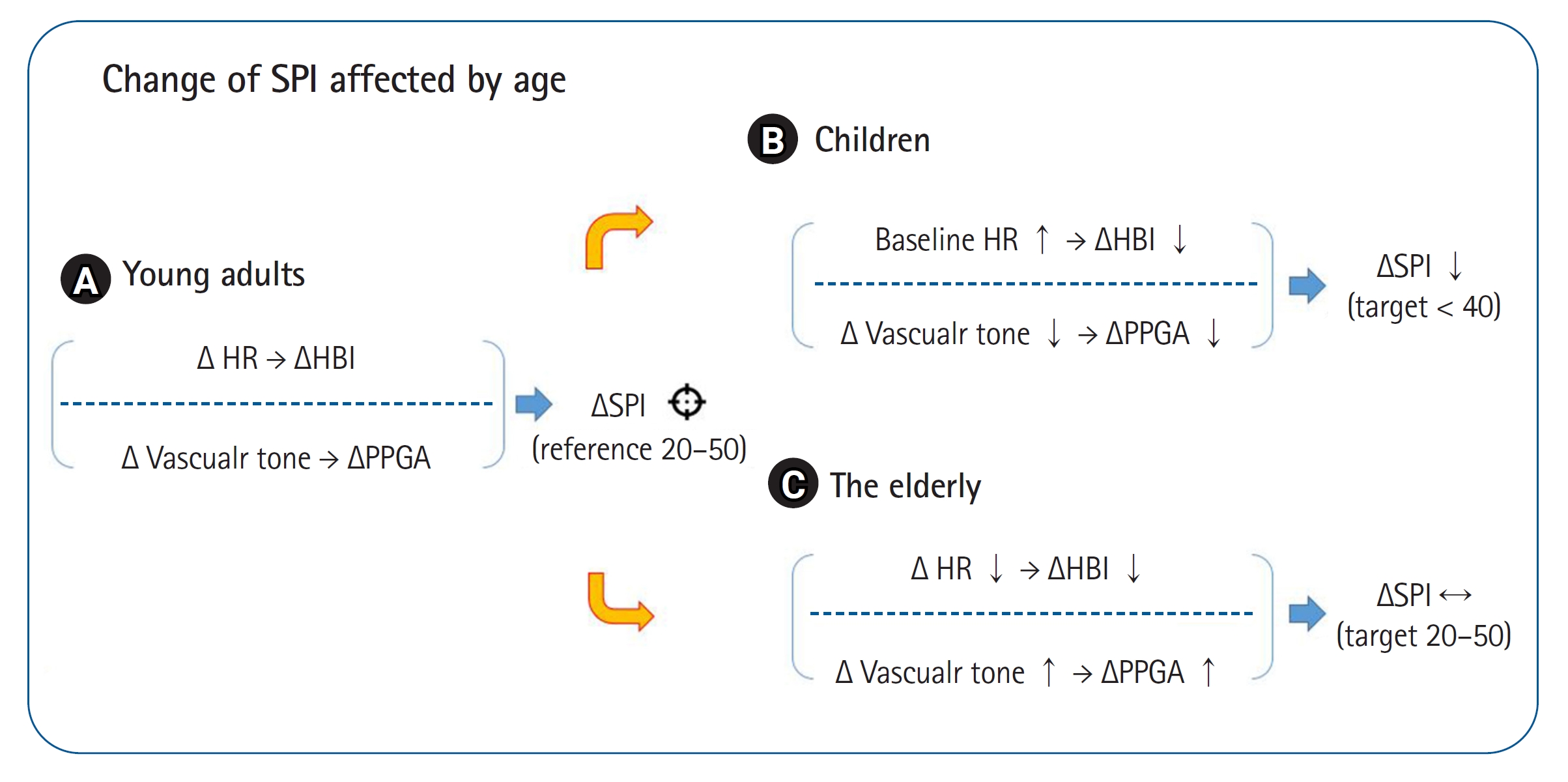
Fig.┬Ā1.

Fig.┬Ā2.
Table┬Ā1.
| Author, Year | Study design | Experimental group (n) | Comparator group (n) | Age range at inclusion (mean or median)* | Type of surgery | Anesthetic/intraoperative opioid | Processed EEG (target) | Primary outcome/main secondary outcomes | Main results and conclusion |
|---|---|---|---|---|---|---|---|---|---|
| Ahonen et al. 2007 [51] | RCT | Esmolol (15) | Remifentanil (15) | 20-65 (32/34) | Gynecological laparoscopic day-care surgery | Desflurane and nitrous oxide/fentanyl | SE (50) | SPI value | While keeping SE at a predetermined level, SPI was higher in patients receiving esmolol than in those receiving remifentanil during laparoscopy. |
| Chen et al. 2010 [5] | Pilot study, RCT | SPI (40) | Conv. (40) | 18-70 (47/46) | ENT surgery expected to last 1 h | Propofol/remifentanil | BIS (40-60) | Opioid consumption/unwanted events, recovery times | SPI resulted in lower remifentanil consumption, more stable hemodynamics, and a lower incidence of unwanted events. |
| Bergmann et al. 2013 [9] | RCT | SPI (76) | Conv. (75) | 18-75 (48/44) | Outpatient orthopedic surgery | Propofol/remifentanil | SE (40-60) | Recovery time, consumption of anesthetics/complications | SPI reduced the consumption of both remifentanil and propofol and resulted in faster recovery. |
| Gruenewald et al. 2014 [21] | RCT | SPI (42) | Conv. (40) | 18-65 (37/41) | Gynecological and orthopedic procedures | Sevoflurane /sufentanil | BIS (40-60) | Unwanted somatic events/hemodynamics, opioid consumption, recovery times | SPI showed no significant differences from standard care in terms of unwanted somatic events, sufentanil consumption, and recovery times. |
| Gruenewald et al. 2015 [58] | Comparative study | SPI (24) | ANI (24: same patients) | 18-65 (40) | Elective surgery | Sevoflurane/remifentanil | BIS (30-60) | Prediction probabilities using receiver operating characteristic for change (Ōłå) in ANI and SPI values | ŌłåANI and ŌłåSPI significantly indicated the patientŌĆÖs movement after tetanic stimulation with a prediction probability of 0.74 and 0.84. Both reflected nociceptive stimulation. |
| Colombo et al. 2015 [22] | RCT | SPI (30) | Conv. (30) | 18-50 (46.6/49.9) | Laparoscopic cholecystectomy | Propofol/remifentanil | SE (40-60) | Sympathetic modulation/hemodynamic variables, opioid consumption, recovery time | SPI led to a more stable sympathetic modulation but did not offer clinically relevant advantages in terms of remifentanil consumption and recovery time. |
| Park et al. 2015 [10] | RCT | SPI (21) | Conv. (24) | 3-10 (7/7) | Adenotonsillectomy | Sevoflurane and nitrous oxide/fentanyl | SE (40-60) | Opioid consumption/sevoflurane consumption, postoperative emergence agitation | SPI does not appear to be valid in children due to differences in blood vessel distensibility and increased baseline heart rates. |
| Won et al. 2016 [12] | RCT | SPI (23) | Conv. (22) | 20-65 (54/42) | Thyroidectomy | Sevoflurane/oxycodone | BIS (40-60) | Opioid consumption/extubation time, NRS | SPI reduced intravenous oxycodone consumption and extubation time compared with conventional analgesia. |
| Won et al. 2017 [19] | RCT | Nicardipine (15) | Remifentanil (15) | 20-65 (46/46) | Thyroidectomy | Desflurane and nitrous oxide | BIS (50) | SPI value | No difference in SPI values between nicardipine and remifentanil. Calcium channel-blocking vasodilators may confound interpretation of the SPI. |
| Jain et al. 2019 [29] | RCT | SPI (68) | Conv. (65) | 18-65 (38.4/40.3) | Laparoscopic cholecystectomy | Sevoflurane/fentanyl | BIS (40-60) | Opioid consumption/hemodynamic changes, recovery time, VAS, PACU analgesia | Intraoperative fentanyl consumption was higher in the SPI group than in controls. Recovery time and hemodynamic changes were comparable. Postoperative VAS and adjuvant fentanyl were higher in controls. |
| Choi et al. 2019 [68] | RCT | Pectoral nerve block type II (20) | Control (19) | 20-65 (52.7/51.4) | Breast surgery | Propofol/remifentanil | BIS (40-60) | Opioid consumption/VAS, PACU analgesia | Pectoral nerve block reduced intraoperative remifentanil consumption by approximately 30% and improved postoperative pain in PACU. |
| Wang et al. 2020 [66] | RCT | Dexmedetomidine (46) | Normal saline (44) | 18-75 (56.8/60.5) | Video-assisted thoracoscopic lung lobectomy | Isoflurane | N/A | SPI and hemodynamic changes/NRS | Dexmedetomidine decreased the intraoperative SPI and NRS scores. Dexmedetomidine attenuated noxious stimuli. |
| Sriganesh et al. 2020 [67] | RCT | Dexmedetomidine (12) | Fentanyl (12) | 18-60 (42.9/42.3) | Craniotomy | Isoflurane | SE (40-60) | SPI changes/biomarkers of surgical stress | No differences were shown in the SPI values or biomarkers, such as cortisol, glucose, and pH, between dexmedetomidine and fentanyl. |
| Kim et al. 2020 [61] | RCT | SPI (43) | Pupillometry (43) | 20-65 (49.4/49.1) | Laparoscopic cholecystectomy | Propofol/remifentanil | SE (40-60) | Peak NRS/opioid consumption | Pupillometry may reduce intraoperative opioid analgesics, recovery room opioid requirements, and pain scores. |
| Funcke et al. 2020 [27] | Pilot study, RCT | SPI (12) | Conv. (12), PPI (12), NOL (12) | Ōēź 18 (64/61/64/62) | Radical retropubic prostatectomy | Sevoflurane/sufentanil | N/A | Opioid consumption/adrenocorticotropic hormone and cortisol | Lower sufentanil in the PPI was associated with an increased endocrine stress response. Titration using the SPI resulted in no reduction in opioid consumption compared to the control but was associated with a reduced endocrine stress response. |
| Funcke et al. 2021 [62] | RCT | SPI (23) | Conv. (24), PPI (24), NOL (24) | Ōēź 18 (62/61/64/62) | Radical retropubic prostatectomy | Propofol/remifentanil | BIS (40-50) | Opioid consumption/adrenocorticotropic hormone and cortisol | Opioid consumption was different for each device (SPI>NOL>PPI). The devices do not seem to be sufficiently validated yet. |
| Gruenewald et al. 2021 [30] | Multicenter, RCT | SPI (246) | Conv. (248) | Ōēź 18 (48/48) | Gynecological, ENT and maxillofacial, orthopedic, trauma | Propofol/remifentanil | SE (40-60) | Unwanted event/eye-open time, PONV, VAS | Entropy and SPI did not reduce adverse events compared with standard monitoring alone. However, there was a reduction in propofol use and shorter emergence time and PACU stay. |
| Guo et al. 2021 [11] | RCT | SPI (31) | Conv. (31) | 18-65 (47.1/48.8) | Laparoscopic cholecystectomy | Propofol/fentanyl | BIS (40-60) | Opioid consumption/extubation time, VAS | SPI lowered intraoperative fentanyl consumption with a shorter extubation time. |
| Stasiowski et al. 2021 [63] | RCT | SPI (31) | Conv. (30), PDR (28) | 18-65 (47.7/49/50.2) | Endoscopic sinus surgery | Propofol/Remifentanil | SE (40-60) | Boezaart bleeding scale | SPI can optimize the condition of the surgical field and reduce blood loss, whereas monitoring based on PDR reduced the use of anesthetic drugs. |
| Muthukalai et al. 2022 [59] | Observational study, plot study | SPI (50) | ANI (50); same patients | 18-60 (40) | Craniotomy | Sevoflurane/fentanyl | SE (40-50) | Bleeding and noradrenaline infusion ŌĆō correlation with the SPI and ANI | The SPI was not affected by bleeding or noradrenaline infusion, contrary to the ANI. |
| Yi et al. 2022 [70] | RCT | Deep NMB (64) | Moderate NMB (64) | 19-85 (63/65) | Laparoscopic herniorrhaphy | Sevoflurane/remifentanil | PSi (25-50) | Opioid consumption/PACU stay | Deep NMB reduced the remifentanil requirement compared with moderate NMB in SPI-guided remifentanil administration for laparoscopic herniorrhaphy. |
| Park et al. 2022 [73] | Observational study | High MELD (Ōēź16) (20) | Low MELD (<16) (20) | 20-70 (58.6/52.1) | Liver transplantation | Isoflurane/remifentanil | BIS (40-60) | Opioid consumption/rescue analgesia | Patients with a higher MELD showed lower remifentanil requirement during surgery but no significant difference during the neo-hepatic phase. |
| Kim et al. 2022 [69] | RCT | Abdominal wall nerve block (24) | Control (28) | 18-70 (57/51) | Inguinal hernia repair | Propofol/remifentanil | SE (40-60) | Opioid consumption/VAS and rescue analgesia | Remifentanil dose during surgery was lower in the nerve block group than the control group when using SPI. |
| Koschmieder et al. 2023 [65] | Observational study | SPI (60) | Conv., PPI, NOL (60; same patents) | Ōēź 18 (42) | Lower extremities surgery | Sevoflurane/sufentanil | BIS (<60) | AUC analyses to predict immediate moderate-to-severe postoperative pain | None of these monitors alone had sufficient diagnostic accuracy to predict early postoperative pain. |
EEG: electroencephalography, RCT: randomized controlled trial, SE: state entropy, ENT: ear nose throat, Conv.: conventional analgesia or standard practice, BIS: bispectral index, ANI: analgesia nociception index, NRS: numerical rating scale for pain, VAS: visual analog scale for pain, PONV: postoperative nausea and vomiting, PACU: post-anesthesia care unit, N/A: data not available, NOL: nociception level, PPI: pupillary pain index, PDR: pupillary dilatation reflex, NMB: neuromuscular blockade, MELD: model for end-stage liver disease, AUC: area under the receiver operating characteristic curve, PSi: patient state index. *Age: age range at inclusion indicates the range indicated in the inclusion criteria, and the values in parentheses are the ages of the included participants.









