 |
 |
| Korean J Anesthesiol > Volume 76(2); 2023 > Article |
|
Abstract
Background
Methods
Results
NOTES
Funding
This work was supported by the Research Institute of Medical Science, Daegu Catholic University, Republic of Korea (No. 201802).
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author Contributions
Eugene Kim (Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Writing – original draft; Writing – review & editing)
Jung A Lim (Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Visualization; Writing – original draft; Writing – review & editing)
Chang Hyuk Choi (Data curation; Formal analysis; Investigation; Methodology; Validation; Visualization; Writing – review & editing)
So Young Lee (Data curation; Formal analysis; Investigation; Methodology; Software; Validation; Visualization; Writing – review & editing)
Seongmi Kwak (Formal analysis; Investigation; Methodology; Software; Validation; Visualization; Writing – review & editing)
Jonghae Kim (Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Software; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing)
Supplementary Materials
Supplementary Marterial 1.
Supplementary Fig. 1.
Supplementary Fig. 2.
Fig. 1.
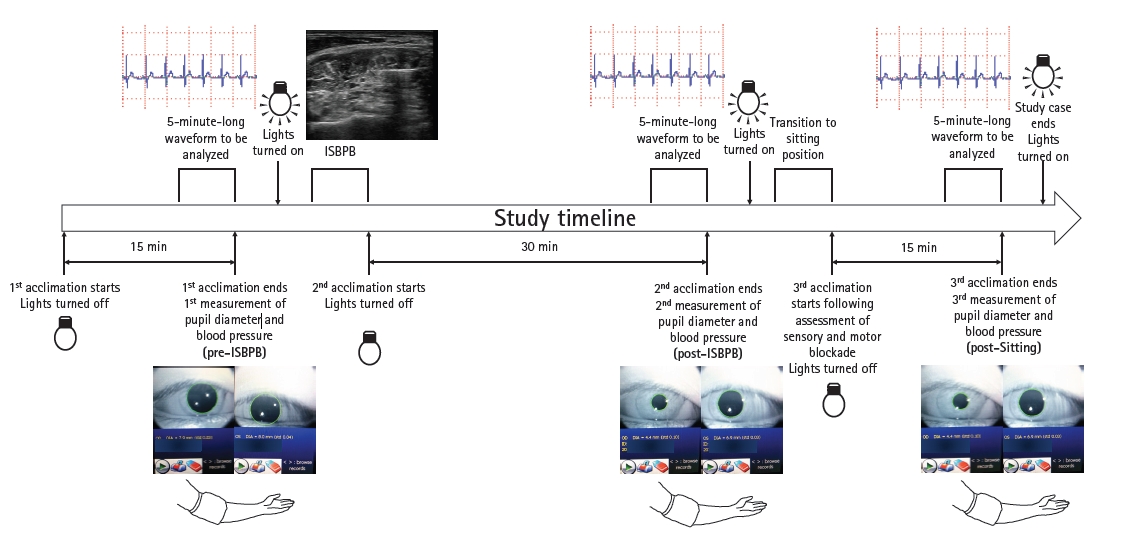
Fig. 2.
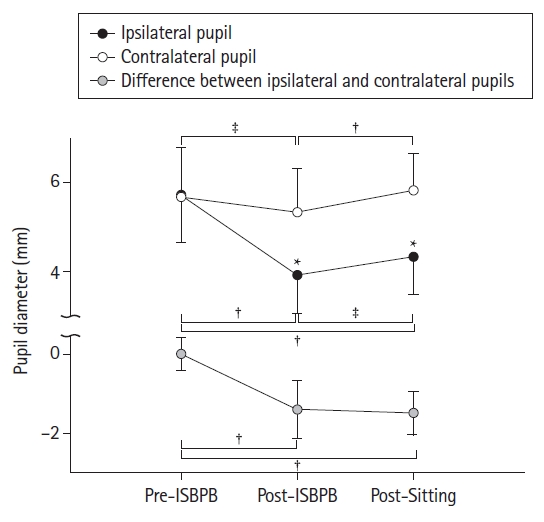
Fig. 3.
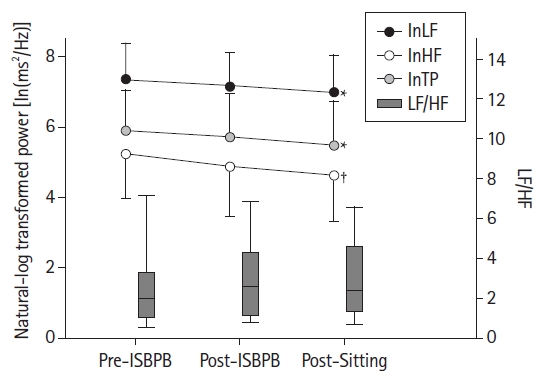
Fig. 4.

Table 1.
Table 2.
Values are presented as median (Q1, Q3) or mean ± SD. *P < 0.05 and †P < 0.01 compared to pre-ISBPB. ISBPB: interscalene brachial plexus block, SDNN: standard deviation of the NN (RR) interval, pNN50: the proportion of the number of interval differences of the successive RR intervals greater than 50 msec in the total number of RR intervals, SDSD: standard deviation of the successive differences of the RR intervals, rMSSD: root mean square of the successive differences of the RR intervals, IRRR: difference between the first and the third quartiles of the successive differences in the RR intervals, MADRR: median of the absolute values of the successive differences in the RR intervals, TINN: the baseline width of the triangular interpolation of NN (RR) interval histogram, HRV: heart rate variability, HRV index: the ratio of total number of RR intervals to the number of RR intervals in a 7.8125 msec-long bin with the most RR intervals, SD1: standard deviation of the points perpendicular to the line of identity in the Poincaré plot, SD2: standard deviation along the line of identity in the Poincaré plot.
Table 3.
References
- TOOLS
-
METRICS

-
- 0 Crossref
- 1 Scopus
- 3,291 View
- 212 Download








