Effect of active airway warming with a heated-humidified breathing circuit on core body temperature in patients under general anesthesia: a systematic review and meta-analysis with trial sequential analysis
Article information
Abstract
Background
The application of a heated-humidified breathing circuit (HHBC) may reduce respiratory heat loss during mechanical ventilation, but its effect in preventing intraoperative hypothermia is controversial. This study aimed to investigate the effectiveness of HHBC in maintaining the core temperature of patients receiving mechanical ventilation under general anesthesia.
Methods
We searched MEDLINE, Embase, Cochrane library (CENTRAL), and Google Scholar to identify all randomized controlled trials (RCTs) up to February 2022 that compared the intraoperative core temperature in patients with heated humidifier (HH) and other circuit devices. The primary outcome was the intraoperative core temperature at the end of surgery. The weighted mean differences (WMDs) between the groups and their 95% CIs were calculated for each outcome. We performed a trial sequential analysis of the primary outcomes to assess whether our results were conclusive.
Results
Eighteen RCTs with 993 patients were included in the analysis. A significantly higher core temperature was observed at the end of surgery in patients with HH than those with no device (WMD = 0.734, 95% CI [0.443, 1.025]) or heat and moisture exchanger (WMD = 0.368, 95% CI [0.118, 0.618]), but with substantial heterogeneity.
Conclusions
Although HHBC did not absolutely prevent hypothermia, this meta-analysis suggests that it can be used as an effective supplemental device to maintain the intraoperative core temperature under general anesthesia. However, considering the substantial heterogeneity and limitations of this study, further well-designed studies are needed to clarify the effectiveness of HHBC.
Introduction
Hypothermia refers to a drop in the core body temperature and is a common problem in anesthetized patients during surgery [1]. After induction of anesthesia, the patient’s core temperature rapidly decreases during the first hour, mainly due to core-to-peripheral redistribution of heat following anesthetic-induced vasodilation. Thermoregulatory impairment due to anesthesia and the cold operating room environment keep these patients hypothermic [2].
Although impairment of thermoregulation is a major factor in hypothermia during general anesthesia, respiratory heat loss also plays a role. When endotracheal intubation is performed in patients under general anesthesia, inspiratory gases become cold and dry due to a lack of heating and humidification in the normal upper airway [3]. Given these physiological changes, the application of a heated humidifier (HH) may prevent respiratory heat loss and help maintain body temperature. A breathing circuit with a built-in heating and humidification system called a heated-humidified breathing circuit (HHBC) has been recently introduced and is widely used in clinical practice for this purpose [4].
As even mild hypothermia can cause serious perioperative complications such as coagulopathy, wound infection, deterioration of cardiovascular function, and delayed recovery time [1,5], preventing temperature decline has always been a major concern in perioperative management. Several studies have investigated the effect of active warming of inspired gases using HH or HHBC for the prevention of intraoperative hypothermia. However, the results vary, and controversy regarding the effectiveness of active airway warming devices still exists [2,6–8].
To the best of our knowledge, no systematic review or meta-analysis has been conducted to summarize or integrate these inconsistent findings. The purpose of this study was to evaluate the effects of active heating and humidification of inhalation gases on the core temperature of patients receiving mechanical ventilation under general anesthesia. It also aimed to provide a basis for the utility of HHBC to healthcare providers and policymakers. By comparing the intraoperative core temperature of patients treated with HH or HHBC to that of patients treated with other circuit devices, we reviewed and synthesized evidences from relevant studies published to date.
Materials and Methods
The systematic review and meta-analysis with trial sequential analysis (TSA) was developed according to the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocol (PRISMA-P) [9]. The protocol of this study was prospectively registered in the PROSPERO network (registration number: CRD42021274160; www.crd.york.ac.uk/prospero) on September 14, 2021. This study was conducted according to the recommendations of the Cochrane Collaboration [10] and reported by observing the PRISMA statement [11]. There are differences between this article and the registered protocol in terms of performance of additional subgroup analysis according to the location of temperature probe (esophageal vs. non-esophageal).
Inclusion and exclusion criteria
The inclusion and exclusion criteria were determined before conducting the study. We included full reports of randomized controlled trials (RCTs) that investigated the effects of active airway warming (HH or HHBC) in patients under general anesthesia.
The PICO-SD information was comprised as follows:
Patients (P): All patients receiving mechanical ventilator care for elective surgery under general anesthesia
Intervention (I): Active airway warming devices including HH and HHBC
Comparison (C): Conventional breathing circuit, passive humidifier (heat and moisture exchange filter), and other airway heating strategies
Outcome measurements (O): The primary outcome of this systematic review and meta-analysis with TSA was the intraoperative core temperature at the end of surgery. The secondary outcomes were the intraoperative core temperature at other time points and pooled combined intraoperative core temperature of multiple time points.
Study design (SD): Full reports of RCTs
Studies involving children (< 18 years of age) or animals, review articles, case reports, case series, letters to the editor, commentaries, proceedings, and any other non-relevant studies were excluded.
Search strategy
Two independent investigators (G.J.C. and H.K.) carried out a comprehensive search in the OVID-MEDLINE, OVID-Embase, Cochrane Central Register of Controlled Trials (CENTRAL), and Google Scholar databases for relevant articles in October 2021 and made final updates by February 2022. Search terms were developed in consultation with a medical librarian and included a combination of free text, Medical Subject Headings, and EMTREE terms including ‘heat,’ ‘humidified,’ ‘circuit,’ and ‘randomized controlled trial.’ The search terms used in MEDLINE and EMBASE are presented in Supplementary Material 1. We also conducted a search of gray literature using OpenSIGLE. Additionally, to ensure that all available studies were searched, we manually scanned the reference lists of the searched original papers until no further relevant studies could be found. We did not apply limitations on the publication date or language.
Study selection
Two investigators (J.J.L. and W.J.L.) independently reviewed the titles and abstracts of the identified studies. If a study was considered eligible on the basis of the title or abstract, the full paper was retrieved and evaluated. Potentially relevant studies identified by at least one investigator or studies with an abstract that could not provide sufficient information regarding the eligibility criteria were retrieved and full-text versions were evaluated. Both investigators discussed their opinions to arrive at a consensus as to whether a study should be included. In cases where a consensus could not be reached, disagreement over inclusion or exclusion was resolved with a discussion with a third investigator (H.K.).
Kappa statistics were used to measure the degree of agreement for study selection between the two independent investigators. Kappa statistics were interpreted as follows: 1) less than 0, less than chance agreement; 2) 0.01 to 0.20, slight agreement; 3) 0.21 to 0.40, fair agreement; 4) 0.41 to 0.60, moderate agreement; 5) 0.61 to 0.80, substantial agreement; and 6) 0.8 to 0.99, almost perfect agreement [12].
Data extraction
Using a standardized data collection form, two independent investigators (J.J.L. and W.J.L.) extracted all interrelated data from the included studies and cross-checked them. When the investigators disagreed, the article was re-evaluated by each investigator until a consensus was reached. If no consensus was reached, a third investigator (H.K.) was consulted.
The following data were extracted: (1) title, (2) name of the first author, (3) name of the journal, (4) year of publication, (5) study design, (6) country, (7) language, (8) risk of bias, (9) type of surgery, (10) inclusion criteria, (11) exclusion criteria, (12) sex, (13) age, (14) number of subjects, (15) any airway heating and humidifying devices and warming manipulations applied intraoperatively, and (16) nature of primary and secondary outcomes investigated.
We selected the core body temperature data if various temperature data were reported in the study. Means and standard deviations of intraoperative core temperatures were initially extracted from tables or text or calculated from the available data, if possible. Data presented only in a graphical format were derived from the open-source software Plot Digitizer (version 2.6.8; http://plotdigitizer.sourceforge.net) [13]. If the values were incomplete or not reported, we attempted to contact the corresponding author to obtain the relevant information.
Risk of bias assessment
Two independent investigators (J.J.L. and S.B.C.) critically appraised the quality of each study using the revised Cochrane risk of bias tool for randomized trials (RoB 2.0 version) [14]. Initially, investigators rated each domain in every study: D1) bias arising from the randomization process, D2) bias due to deviations from the intended interventions, D3) bias due to missing outcome data, D4) bias in measurement of the outcome, and D5) bias in selection of the reported result. The overall risk of bias was also evaluated. It was judged as low risk when the risk of bias for all domains was low; high, when the risk of bias for at least one domain was high or the risk of bias for multiple domains was of some concern; and some concern, if the overall judgment was neither low nor high. In cases where a consensus could not be reached, disagreement was resolved by discussion with a third investigator (H.K.).
Data analysis
Conventional meta-analysis
Meta-analysis was conducted using Comprehensive Meta-Analysis version 2.0 (Englewood, NJ, USA, 2008). Two investigators (J.J.L. and W.J.L.) independently inputted all data into the software. The weighted mean difference (WMD) and 95% CI were calculated for each outcome.
We used the chi-square and I2 tests to explore the heterogeneity between the studies [15]. A Pchi2 less than 0.1 or an I2 greater than 50% was considered to indicate considerable heterogeneity. A fixed effects model was selected if Pchi2 ≥ 0.10 and I2 ≤ 50%. In cases of Pchi2 < 0.10 or I2 > 50%, a random effects model was used [16].
To explore heterogeneity, we performed a sensitivity analysis by removing one study at a time and determining whether it altered the results.
Subgroup analyses were performed according to the location of the temperature probe (esophageal vs. non-esophageal).
Publication bias was assessed using Begg’s funnel plot and Egger’s linear regression test, and a P value < 0.05 was used to identify the presence of a publication bias; otherwise, funnel plots for each data set were visually assessed for asymmetry. If a publication bias was present, a trim-and-fill analysis was performed to evaluate its effect [17].
TSA
We additionally performed a TSA on the intraoperative core temperature at the end of surgery using TSA software (Copenhagen Trial Unit, Centre for Clinical Intervention Research, Denmark) to assess whether the results of the conventional meta-analysis were conclusive. Conventional meta-analysis runs the risk of overestimation (type I errors) or underestimation (type II errors) owing to sparse data [18]. TSA provides more information on the precision and uncertainty of meta-analysis results, specifically the required information size (RIS) and a threshold for statistical significance, which controls the risk of potential false-positive and false-negative findings of meta-analyses. We used a random effects model with the DerSimonian–Laird (DL) method to construct the cumulative Z-curve. TSA was performed to maintain an overall 5% risk of a type I error.
When the cumulative Z-curve crossed the trial sequential monitoring boundary or entered the futility area, a sufficient level of evidence to accept or reject the anticipated intervention effect may have been obtained, and no further studies were needed. If the Z-curve did not cross any boundaries and the RIS was not reached, the evidence to reach a conclusion would be considered insufficient, indicating the need for further studies.
We used an alpha of 5% for all outcomes, a beta of 10%, and the observed mean difference, variance, and diversity, as suggested by the trials in the meta-analysis.
Quality of the evidence
Evidence grade was determined using the guidelines of the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system, which uses a sequential assessment of evidence quality, followed by an assessment of the risk-benefit balance and a subsequent judgment on the strength of the recommendations [19].
Results
Study selection
A total of 837 articles were acquired after searching the databases, and 11 additional articles were identified after conducting a manual search (Fig. 1). After excluding duplicates (n = 95), 753 articles were obtained. We reviewed the titles and abstracts of these articles, and 719 did not meet the selection criteria. In this stage of study selection, the kappa value for selecting articles between the two investigators was 0.798.
The full text of the remaining 34 articles was reviewed in more detail, and another 16 were excluded for the following reasons: review article [20,21], non-human study [22], case series [23], non-randomized study [24,25], no subject of interest [26], no intervention of interest [27], unsuitable control group [28], and no outcome of interest [29–35]. The kappa value for selecting articles between the two investigators was 0.820. Finally, 18 relevant studies with a total of 993 patients were included in our systematic review and meta-analysis with TSA.
Study characteristics
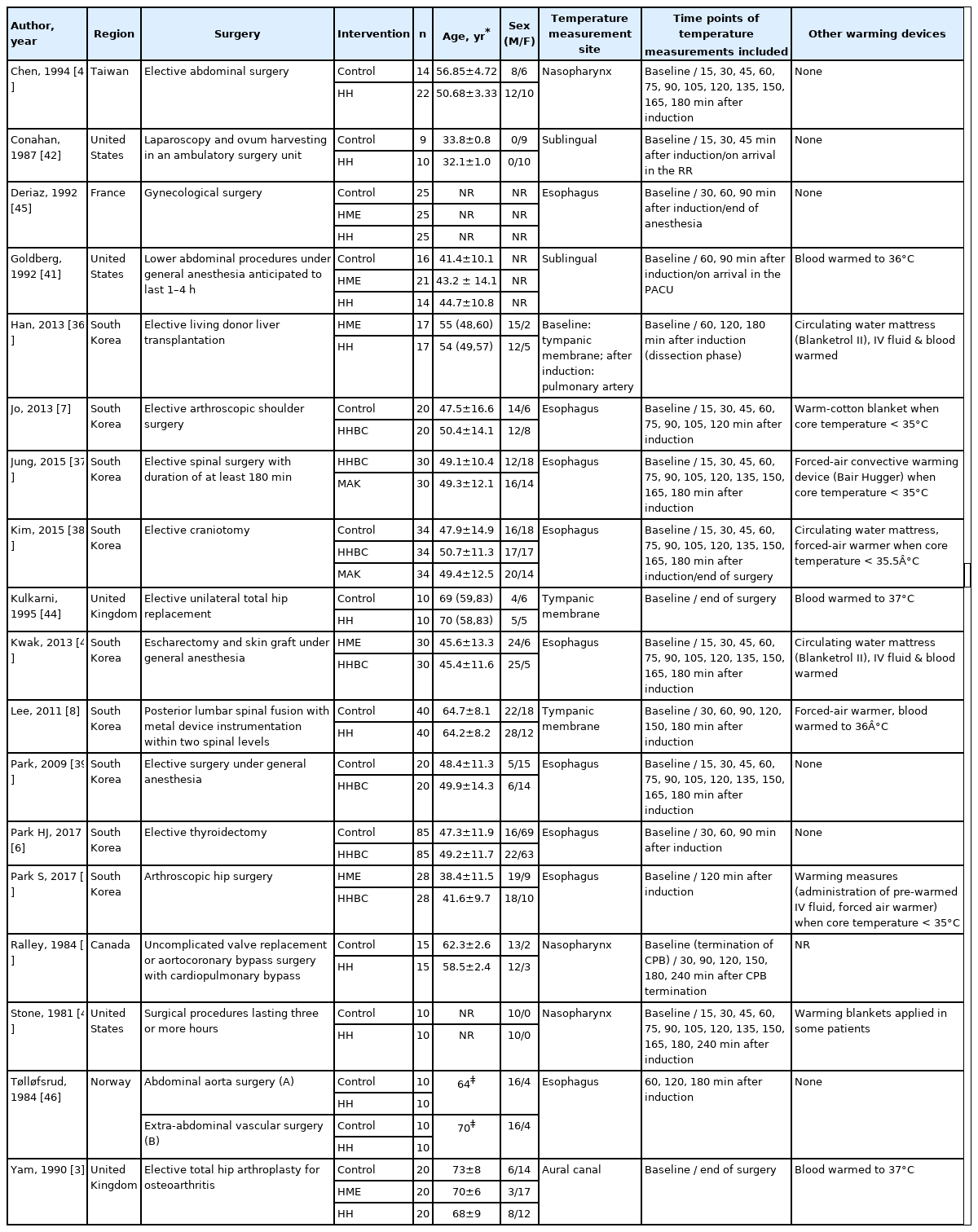
The study characteristics are summarized in Table 1. All selected studies were prospective randomized clinical trials. Classifying these studies by country where the study was conducted, South Korea had the largest number of studies with nine articles [2,6–8,36–40], followed by the United States with three articles [41–43], United Kingdom with two articles [3,44], and France [45], Norway [46], Canada [47], and Taiwan [48] with one article each. All included patients received general anesthesia and mechanical ventilator care for their scheduled operations. The types of surgery the patients had in the included studies are as follows: abdominal surgery [36,41,48], gynecological surgery [42,45], orthopedic surgery including arthroscopic surgery and arthroplasty [2,3,7,44], spinal surgery [8,37], neurosurgery [38], thyroid surgery [6], escharectomy and skin graft for patients with major burn [40], uncomplicated cardiac surgery with cardiopulmonary bypass (CPB) [47], vascular surgery [46], and unspecified elective surgery [39,43]. In one study of patients undergoing liver transplantation surgery [36], only core temperature data up to 3 h after anesthesia induction (before the extraction of liver) were included in the analysis, because the operation process, such as liver extraction or reperfusion, can greatly affect the patient’s body heat content. One study of patients undergoing cardiac surgery using CPB [47] reported the core body temperature measured from CPB weaning to the end of surgery. Considering that it was not directly affected by extracorporeal circulation, we included the temperature data reported in this study.
For active warming and humidification of inspired gas, HH was applied to the inspiratory limb of the anesthetic breathing circuit in 11 studies [3,8,36,41–48] and HHBC was used in seven studies [2,6,7,37–40]. In the analysis, interventions compared to HH (including HHBC) were as follows: control without any other airway heating devices [3,6–8,38,39,41–48], heat and moisture exchanger (HME) filter [2,3,36,40,41,45], and Mega Acer Kit (MAK), an HHBC including a fluid warming device [37,38]. In these two studies, as the infused volume of intravenous fluid might affect the patient’s temperature change [1], the infusion rate of fluid was constantly managed among the study [37] or it was confirmed that there was no statistical difference in the total volume of IV fluid infused between the groups [38].
The measurement sites for monitoring the patient’s intraoperative core body temperature were the esophagus [2,6,7,37–40,45,46], tympanic membrane [3,8,36,44], nasopharynx [43,47,48], and pulmonary artery [36]. One study measured the baseline core temperature via the tympanic membrane and recorded the pulmonary arterial temperature after anesthesia induction [36]. Two studies extracted the sublingual temperature data [41,42]. In these two studies, the core body temperature data were either insufficient or not reported. Our attempt to contact the authors and request the core body temperature data was unsuccessful. Therefore, we included these data in our pooled analysis and performed a sensitivity analysis, excluding the data from the two studies.
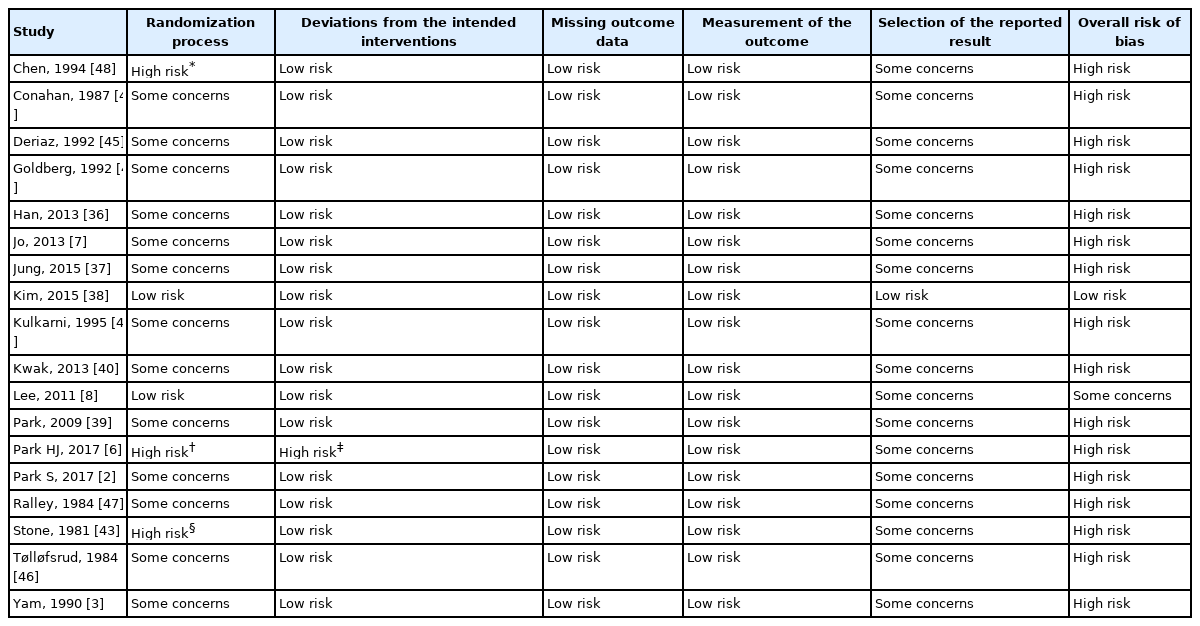
Risk of bias assessment
The overall risk of bias assessment is presented in Table 2. For the overall risk of bias, 16 studies were evaluated to be high risk. One study evaluated to be low risk and one study as some concerns. Bias arising from the randomization process was assessed to be high risk in three studies and some concerns in 13 studies. With the exception of one study [38], allocation concealment was not described in most studies. One study used a computer-generated random table 15 min before induction [8]; thus, allocation concealment was performed. One study randomly assigned patients according to the expected duration of surgery [43]; it was suspected that allocation concealment could not be achieved. For 17 studies with no information on a pre-specified analysis plan or trial registration, bias arising from selection of the reported results was judged as having some concerns. One study [6] was judged as high risk in two bias domains. In this study, all dropouts occurred in the control group, and to make the groups comparable, investigators matched the patients in the control group to those in the HHBC group according to their age. The final analysis was performed only for matched patients. Therefore, the study was judged as high risk due to the randomization process and deviations from the intended interventions.
Conventional meta-analysis
HH vs. Control
Fifteen studies comparing core body temperature in 683 patients under general anesthesia receiving mechanical ventilation with HH or HHBC and in the control group (patients without inhalation gas warming devices) were evaluated in the analysis. At baseline, the difference in the core temperature between the HH and control groups was insignificant (WMD = 0.054, 95% CI [–0.028, 0.135], I2 = 61.09, τ2 = 0.09, P = 0.015) (Supplementary Fig. 1).
Intraoperative core temperature at the end of surgery. The primary outcome of this meta-analysis was the intraoperative core temperature at the end of surgery. From the studies that did not report the temperature measured at the end of surgery, the last reported core temperature value was included in the analysis. The HH group showed a significantly higher core temperature at the end of surgery than the control group, but with substantial heterogeneity (WMD = 0.734, 95% CI [0.443, 1.025], I2 = 96.65, τ2 = 0.31, P < 0.001) (Fig. 2A). The subgroup analysis for the location of the temperature probe showed that the core temperature at the end of surgery was significantly higher in the HH group than in the control group for both the esophageal temperature (WMD = 0.432, 95% CI [0.274, 0.590], I2 = 65.82, τ2 = 0.23, P = 0.007) and non-esophageal temperature subgroups (WMD = 1.011, 95% CI [0.494, 1.528], I2 = 98.10, τ2 = 0.54, P < 0.001).
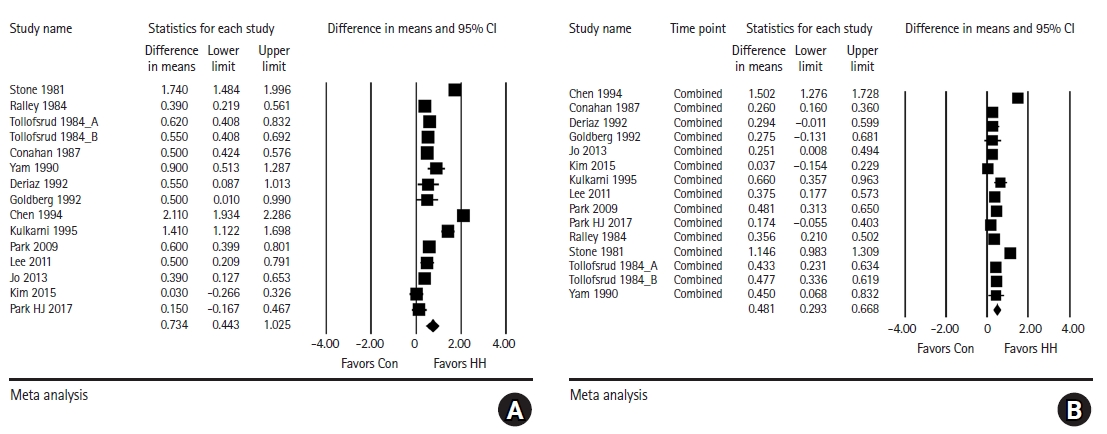
Forest plot for analysis comparing the intraoperative core temperature (°C) between the HH group and control group. (A) At the end of surgery, (B) pooled combined intraoperative core temperature of multiple time points. The core body temperature at the end of surgery and pooled combined core body temperature are significantly higher in the HH group than in the control group. HH: heated humidifier.
Mean difference of intraoperative core temperature over time: The trend of core body temperature during anesthesia in both groups is shown in Fig. 3. A decrease in the core body temperature after induction was observed in both groups, but the decrease was less in the HH group than in the control group. The HH group showed a tendency to maintain body temperature from approximately 1 h after induction. The intraoperative core temperature was significantly higher in the HH group than in the control group at all time points at 15 min intervals after anesthesia induction (Fig. 3). In addition, the difference between the two groups tended to increase over time after induction. However, at all points, I2 was greater than 50% and Pchi2 was less than 0.1, indicating substantial heterogeneity (Supplementary Material 2, Supplementary Fig. 2).
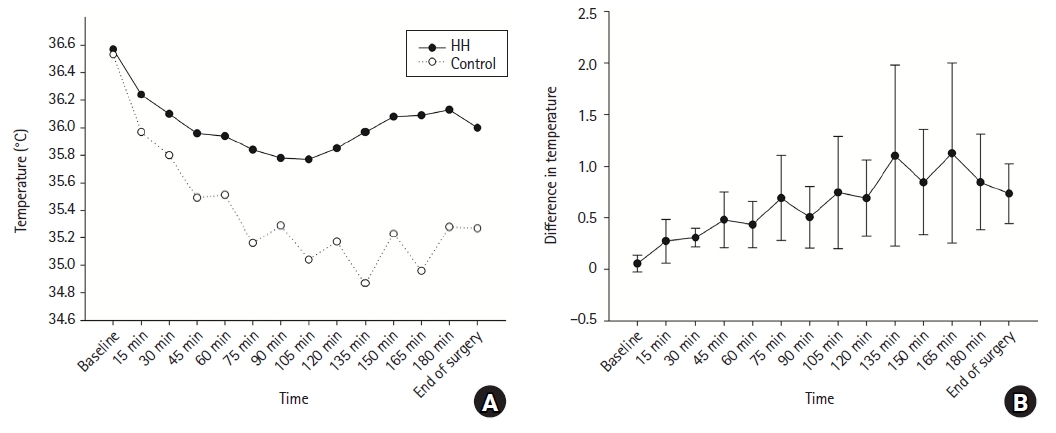
The intraoperative core temperature (°C) over time in the HH group and control group (A) and the mean difference between the two groups (B). Temperature decline after induction of anesthesia is lesser in the HH group. Core body temperature during anesthesia is significantly higher in the HH group compared to the control group. HH: heated humidifier.
Pooled combined intraoperative core temperature of multiple time points: The average core temperature during anesthesia in the included studies was compared between the two groups. The pooled combined core body temperature value was significantly higher in the HH group than in the control group, with a mean difference of 0.481°C; however, substantial heterogeneity was detected (95% CI [0.293, 0.668], I2 = 92.97, τ2 = 0.12, P < 0.001) (Fig. 2B). The subgroup analysis for the location of temperature probe showed that the pooled combined intraoperative core temperature was significantly higher in the HH group than in the control group for both esophageal temperature (WMD = 0.316, 95% CI [0.179, 0.453], I2 = 68.99, τ2 = 0.23, P = 0.004) and non-esophageal temperature (WMD = 0.633, 95% CI [0.303, 0.963], I2 = 98.10, τ2 = 0.21, P < 0.001).
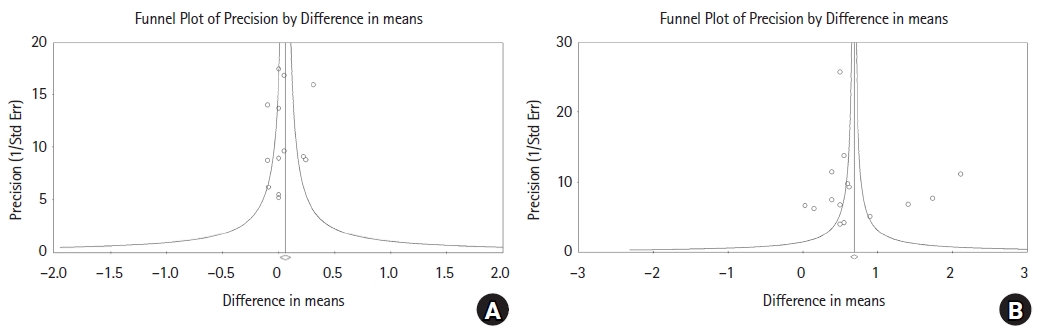
Publication bias: Begg’s funnel plots are shown in Fig. 4 and Supplementary Fig. 3. For baseline data among the included studies, the funnel plot was symmetric and Egger’s test also showed insignificant results (Coef = -0.0512, 95% CI [–3.326, 2.303], P = 0.697). The P values from Egger’s test for data at 30, 60, and 90 min post-induction and at the end of surgery and for the overall data were all greater than 0.05, indicating that no evidence of publication bias was detected.
HH vs. HME
Six studies were selected, including 275 patients, to compare the core body temperature between patients with HH/HHBC and patients with HME filters. The difference in the core temperature between the HH and HME groups was negligible at baseline (WMD = 0.066, 95% CI [–0.035, 0.167], I2 = 36.99, τ2 = 0.01, P = 0.161) (Supplementary Fig. 4).
Intraoperative core temperature at the end of surgery: The results of the analysis are shown in Fig. 5A. Core temperature at the end of surgery was significantly higher in the HH group than in the HME group, but with substantial heterogeneity (WMD = 0.368, 95% CI [0.118, 0.618], I2 = 74.60, τ2 = 0.07, P = 0.060). The subgroup analysis for the location of temperature probe showed that the core temperature at the end of surgery was significantly higher in the HH group than in the HME group for esophageal temperature (WMD = 0.361, 95% CI [0.115, 0.607], I2 = 53.06, τ2 = 0.03, P = 0.119), but not for non-esophageal temperature (WMD = 0.394, 95% CI [–0.134, 0.922], I2 = 86.90, τ2 = 0.19, P < 0.001).
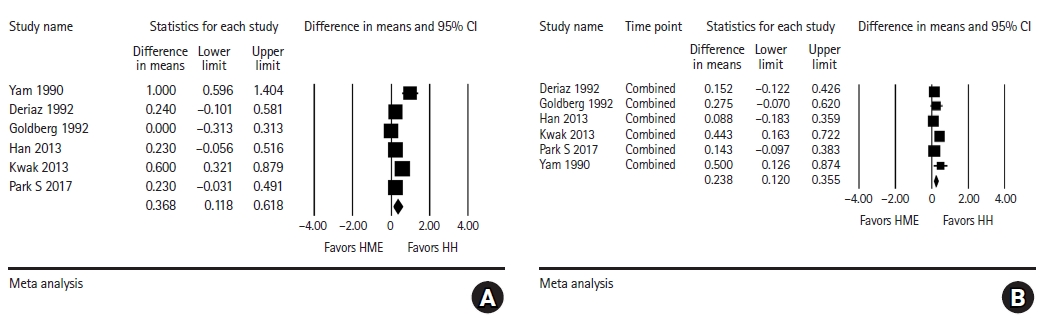
Forest plot for analysis comparing the intraoperative core temperature (°C) between the HH group and HME group. (A) At the end of surgery, (B) pooled combined intraoperative core temperature of multiple time points. The core body temperature at the end of surgery and pooled combined core body temperature are significantly higher in the HH group than in the HME group. HH: heated humidifier, HME: heat and moisture exchanger.
Mean difference of intraoperative core temperature over time: As shown in Fig. 6, a significant difference in the intraoperative core temperature between the HH and HME groups was observed at all time points except at 60 min after induction (WMD = 0.263, 95% CI [–0.026, 0.553], I2 = 76.89, τ2 = 0.07, p = 0.005). In general, the core temperature during anesthesia was significantly higher in the HH group, and the difference between the two groups tended to increase slightly over time. However, considerable heterogeneity was detected at 60, 120, and 180 min after induction (Supplementary Material 2, Supplementary Fig. 4).

Mean difference in intraoperative core temperature (°C) between the HH group and HME group over time. Intraoperative core temperature is significantly higher in the HH group, except at 60 min after induction. HH: heated humidifier, HME: heat and moisture exchanger.
Pooled combined intraoperative core temperature of multiple time points: The overall intraoperative core temperature was higher in the HH group than in the HME group, with a significant mean difference of 0.238°C (95% CI [0.120, 0.355], I2 = 18.55, τ2 = 0.01, P = 0.293), as shown in Fig. 5B. The subgroup analysis for the location of temperature probe showed that the pooled combined intraoperative core temperature was significantly higher in the HH group than in the HME group for both esophageal temperature (WMD = 0.234, 95% CI [0.083, 0.386], I2 = 34.15, τ2 = 0.09, P = 0.219) and non-esophageal temperature (WMD = 0.260, 95% CI [0.058, 0.428], I2 = 35.41, τ2 = 0.15, P = 0.213).
HH vs. MAK
Two studies with a total of 128 patients compared the intraoperative core body temperature between patients with HH/HHBC and patients with MAK units. At baseline, the core body temperature was 0.111°C higher in the MAK group than in the HH group (95% CI [–0.204, –0.018], I2 = 0.00, τ2 = 0.00, P = 0.658) (Supplementary Fig. 5).
Intraoperative core temperature at the end of surgery: Although the core body temperature of the MAK group at the end of surgery was 0.601°C higher than that of the HH group, the difference between the two groups was not statistically significant (95% CI [–1.238, 0.036], I2 = 95.27, τ2 = 0.20, P < 0.001) (Fig. 7A).
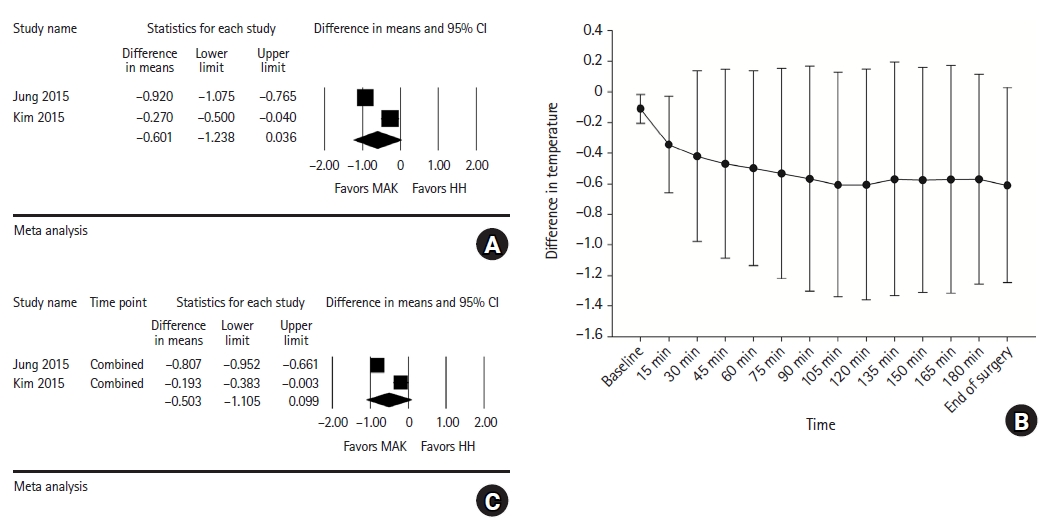
Forest plot for analysis comparing the intraoperative core temperature (°C) at the end of surgery (A) and pooled combined intraoperative core temperature of multiple time points (C) between the HH and MAK groups. Mean difference of the intraoperative core temperature (°C) between the HH and MAK groups over time (B). HH: heated humidifier, MAK: Mega Acer Kit.
Mean difference in intraoperative core temperature over time: The difference in the intraoperative core body temperature between the HH and MAK groups is shown in Fig. 7B. From baseline to 15 min after anesthesia induction, the core temperature of the MAK group was significantly higher than that of the HH group (at 15 min after induction, WMD = –0.343, 95% CI [–0.656, –0.029], I2 = 81.99, τ2 = 0.04, P = 0.010). Although the intraoperative core temperature was consistently higher in the MAK group and the mean value of the difference between the two groups tended to initially increase and stabilize over time, the difference from 30 min after induction to the end of surgery was not statistically significant (Fig. 7B, Supplementary Material 2, Supplementary Fig. 5).
Pooled combined intraoperative core temperature of multiple time points: Despite the higher overall intraoperative core temperature in the MAK group, the difference in the pooled combined core temperature between the two groups was statistically insignificant (WMD = –0.503, 95% CI [–1.105, 0.099], I2 = 96.05, τ2 = 0.18, P < 0.001) (Fig. 7C).
Sensitivity analysis
Sensitivity analysis was conducted with the exclusion of one study at a time, and there was no change in statistical significance.
TSA
HH vs. Control
As shown in Fig. 8A, TSA indicated that more patients (528) than the RIS (173) were accrued. The cumulative Z-curve (complete blue curve) crossed both the conventional test boundary (etched red line) and trial sequential monitoring boundary (complete red curve).
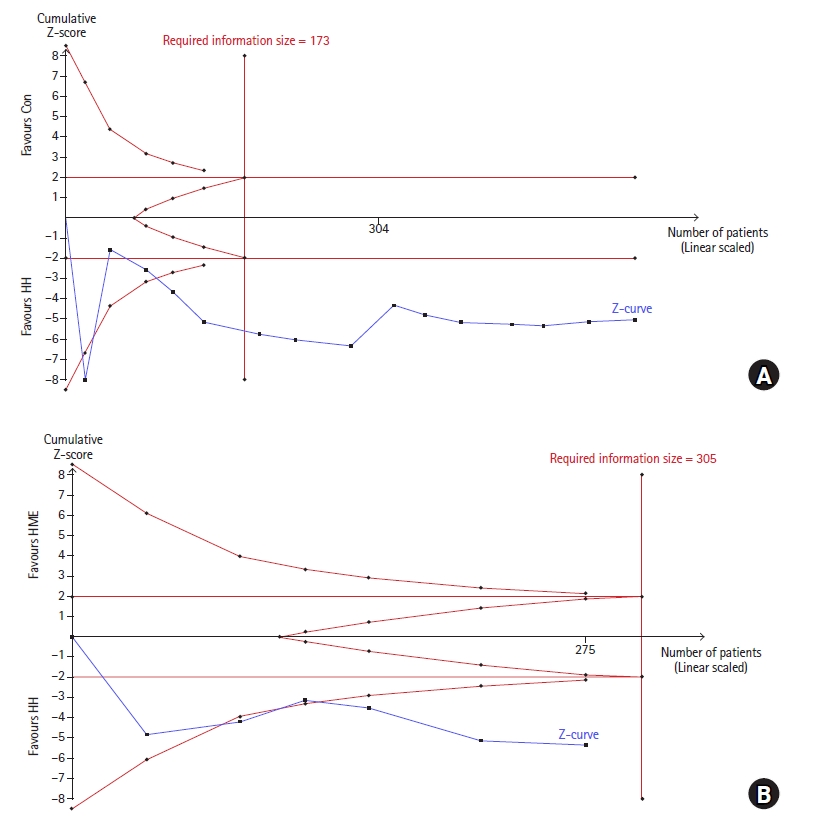
TSA on the intraoperative core temperature at the end of surgery. (A) HH vs. control and (B) HH vs. HME. (A) The cumulative Z-curve (complete blue curve) passed the RIS (vertical red line) and crossed both the conventional test boundary (etched red line) and trial sequential monitoring boundary (complete red curve). (B) The cumulative Z-curve did not reach the RIS. It crossed both the conventional test boundary and trial sequential monitoring boundary. TSA: trial sequential analysis, HH: heated humidifier, HME: heat and moisture exchanger, RIS: required information size.
HH vs. HME
TSA indicated that only 90.2% (275 of 305 patients) of the RIS was accrued. The cumulative Z-curve (complete blue curve) crossed both the conventional test boundary (etched red line) and trial sequential monitoring boundary (complete red curve) (Fig. 8B).
HH vs. MAK
The cumulative Z-curve (complete blue curve) did not reach the RIS, and only 30.5% (128 of 419 patients) of the RIS was accrued. The Z-curve crossed both the conventional test boundary (etched red line) and trial sequential monitoring boundary (complete red curve), but returned to be within the conventional test boundary (Supplementary Fig. 6).
Discussion
This systematic review and meta-analysis was designed to investigate whether active warming and humidification of inhaled gases helps maintain the core body temperature of patients receiving mechanical ventilation under general anesthesia. Although the application of HH or HHBC did not completely prevent hypothermia, our meta-analysis indicates that using active airway warming devices can attenuate the initial decline in intraoperative core temperature and contribute to maintaining core temperature from approximately 1 h after induction of anesthesia.
During general anesthesia, a rapid decrease in core temperature due to core-to-peripheral redistribution occurs until the first hour after anesthesia induction. Subsequently, a slower reduction in the core temperature appears for the next 2 to 4 h. It is mainly caused by heat loss through radiation, convection, conduction, and evaporation exceeding the metabolic heat production [1]. Respiratory heat loss accounts for approximately 10% of metabolic heat production under normal conditions. While radiation accounts for the largest portion of intraoperative heat loss, respiratory heat loss accounts for a relatively small portion [8]. HH and HHBC supply heat and moisture to the inhalation gas by using an external source of heat energy and water. Reducing evaporation in the respiratory mucous membrane and some of the heat convection are supposed to be the mechanisms for body heat preservation of the HH [7]. Therefore, the use of HH or HHBC may have a limited effect in compensating for intraoperative heat loss and preventing hypothermia.
As a result of the meta-analysis, compared to the HME filter, HHBC was found to be more advantageous in maintaining intraoperative core body temperature. HME performs the action of heating and humidification, similar to the nasal cavity. It retains heat and moisture from every exhaled breath with a condenser and returns them back to the next inspired gas in a passive manner [20]. Considering the limited source of heat and moisture in the HME filter, it is believed that, comparatively, HH would have a greater effect on core body temperature [36]. In addition, the results of TSA support the results of our conventional meta-analysis that HH and HHBC are more effective in preserving core body temperature during anesthesia than a HME filter or conventional breathing circuit. In the meta-analysis comparing HH and MAK, the differences in core temperature between the two groups were not statistically significant, but consistently higher mean temperatures were observed in the patients with MAK, which is an HHBC that includes an IV fluid warming kit. This suggests that HH may be more effective when used in combination with other warming devices. Considering the overall results of this study, we recommend that HHBC should be used in combination with other warming manipulations to reduce the incidence of intraoperative hypothermia.
Although a significantly higher intraoperative core temperature was observed in patients with HH/HHBC than in those with a conventional breathing circuit or HME, the possibility of measurement error cannot be ruled out. Bissonnette et al. [49] reported that when airway heating was applied, the esophageal temperature was approximately 0.35°C above the tympanic membrane temperature. In this study, half of the included studies reported core temperature data measured in the esophagus, which is susceptible to direct influence of a HH due to its anatomy. However, the subgroup analyses demonstrated that the location of the temperature probe had little effect on the result that the core body temperature was significantly higher in the HH group than in the control or HME group.
The present study has several limitations. Substantial heterogeneity was observed in the results. There were several possible sources of clinical and methodological heterogeneity among the included studies, such as the type and duration of surgery, demographic characteristics of patients, measurement site and timing of temperature, anesthetic management, and perioperative thermal manipulations. Although we performed a sensitivity analysis, we could not control for heterogeneity. Second, the quality of the included RCTs was limited. The overall risk of bias was high in a significant number of the included studies. Third, the effect on intraoperative core body temperature was the only outcome evaluated in our study to determine the effectiveness of HH. Among the studies evaluated, data on other outcomes that could determine the effectiveness of HH, such as postoperative shivering, laryngopharyngeal complaints, and postoperative respiratory complications, were insufficient to perform a meta-analysis. In addition, we could not provide any information regarding the cost-effectiveness of HH or HHBC. HHBC devices are much more expensive than conventional breathing circuits and HME filters. To determine the recommendation of using any medical equipment, it is essential to weigh the cost of utilization and following medical advantages, as well as patient satisfaction.
Based on the limitations of this study, additional well-designed studies with a low risk of bias are needed to confirm the effectiveness of HHBC suggested by our analysis. Furthermore, future studies on specific populations, such as children and the elderly, or under specific surgical conditions, would make it possible to investigate the indicators that can benefit significantly from the use of HHBC.
Despite these limitations, this study had several strengths. First, this is the first systematic review and meta-analysis to examine the effects of airway heating and humidification using HHBC on intraoperative body temperature in patients under general anesthesia. A rigorous methodology was applied to the study based on a registered, pre-planned protocol. Second, the effect of HHBC was compared with various anesthetic circuit devices, including the HME filter and MAK, as well as with a control using a conventional breathing circuit. Third, to control for the possibility of a type I error from multiple comparisons at each time point, we combined the intraoperative core temperature data of multiple time points and performed additional analysis using the pooled combined outcomes. Finally, we performed a TSA to evaluate the statistical significance and strength of the available evidence, despite considerable heterogeneity.
In conclusion, the current study suggests that HHBC can be used as an effective supplemental device to maintain core body temperature during surgery under general anesthesia. Although active airway warming and humidification cannot absolutely prevent hypothermia, the use of HH or HHBC is more effective in attenuating the temperature decline and maintaining a higher intraoperative core temperature than conventional breathing circuit or HME filter. However, considering the substantial heterogeneity and limitations of this study, additional data from well-designed studies are needed to clarify the effectiveness of HHBC and improve the quality of evidence.
Notes
Funding
None.
Conflicts of Interest
Kang Hyun was an Editor for the Korean Journal of Anesthesiology (KJA) from 2020 to 2022 and Geun Joo Choi has also been an editor for the KJA since 2020. However, they were not involved in any process of review for this article, including peer reviewer selection, evaluation, or decision-making. There were no other potential conflicts of interest relevant to this article.
Data Availability
The study data supporting this systematic review and meta-analysis were obtained from previously reported studies and datasets, which have been cited. The data presented in this study are available in this article and Supplementary Materials.
Author Contributions
Je Jin Lee (Conceptualization; Data curation; Formal analysis; Investigation; Visualization; Writing – original draft; Writing – review & editing)
Geun Joo Choi (Formal analysis; Investigation; Methodology; Writing – review & editing)
Won Jun Lee (Data curation; Investigation)
Sang Bong Choi (Data curation; Visualization)
Hyun Kang (Conceptualization; Data curation; Formal analysis; Methodology; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing)
Supplementary Materials
Search terms used in literature search.
Supplementary data.
Forest plot for analysis comparing the intraoperative core temperature (°C) at baseline between the HH group and control group.
Forest plots for analysis comparing the intraoperative core temperature (°C) over time between the HH group and control group.
Begg’s funnel analysis comparing the HH group and control group for data of the pooled combined intraoperative core temperature at multiple time points.
Forest plots for analysis comparing the intraoperative core temperature (°C) over time between the HH group and HME group.
Forest plots for analysis comparing the intraoperative core temperature (°C) over time between the HH group and MAK group.
TSA comparing intraoperative core temperature at the end of surgery. HH vs. MAK.