Non-convulsive status epilepticus in the immediate postoperative period following spine surgery -a case report-
Article information
Abstract
Background
Non-convulsive status epilepticus (NCSE), in which continuous epileptiform discharges occur without seizure-like movement, is rare and unfamiliar to anesthesiologists, both of which make this condition overlooked in patients with decreased levels of consciousness following general anesthesia.
Case
We report on an elderly female patient who developed NCSE in the immediate postoperative period after the spine surgery. Initially, delayed emergence from anesthesia was suspected, but the electroencephalogram confirmed NCSE, and anticonvulsant therapy was initiated.
Conclusions
Delayed emergence is commonly attributed to cerebrovascular events or residual anesthetic effects, but NCSE must be included in the differential diagnosis, especially in elderly patients. Anticonvulsant therapy should be initiated as soon as possible for a better prognosis.
Non-convulsive status epilepticus (NCSE) can be defined as the condition of continuous or intermittent clinical epileptic activity without convulsion, lasting at least for 30 minutes with electroencephalogram (EEG) evidence of seizure [1]. The main clinical feature of NCSE is a change in behavior or consciousness such as mutism, mild amnesia to stupor, and agitation [1,2]. However, there is no universally accepted definition of NCSE.
A decreased level of consciousness after general anesthesia could be attributed to residual anesthetic effects, cerebrovascular events such as hemorrhage or ischemia, and other metabolic derangements. Therefore, it is not easy to distinguish NCSE among the many possible causes of decreased consciousness or lack of responsiveness. The occurrence of NCSE in the immediate postoperative period is too rare to estimate the prevalence. We found only two reported cases of NCSE in the immediate postoperative period after the brain surgery [3,4]. A single report from India documented two patients with NCSE after the extracranial surgery. However, the patients were thought to have NCSE based on circumstantial evidence, not EEG findings [5]. To our knowledge, the presentation of NCSE immediately after the non-cranial surgery has never been published.
The following case describes an elderly female patient who developed NCSE in the immediate postoperative period and presents a discussion that highlights the importance of NCSE in the differential diagnosis of decreased consciousness.
Case Report
The Institutional Review Board of Dongguk University Ilsan Hospital approved this case report (approval number: 2020-09-022). A 75-year-old woman with well-controlled hypertension and diabetes mellitus (height: 154.1 cm, weight: 52.8 kg) was scheduled to undergo a transforaminal lumbar interbody fusion of L3-S1. She had undergone uneventful surgery with general anesthesia for a laminectomy eight years earlier. She was taking oral hypoglycemic and antihypertensive agents with analgesics for back pain. She had no history of psychiatric illnesses or drug addiction. Her preoperative vital signs and biochemical parameters were unremarkable. General anesthesia was induced with propofol and maintained with air, oxygen, desflurane, remifentanil, and muscle relaxation with vecuronium. A mean arterial pressure (MAP) was maintained around 75 mmHg by an intermittent bolus injection of ephedrine, and her MAP was never below 55 mmHg at any point during the surgery. There was no episode of hypoxia, and the peripheral oxygen saturation was 100% throughout the surgery. The body temperature measured with an esophageal stethoscope was 36.1℃ at the beginning of the surgery and gradually decreased even though an air warmer was applied, and at the end of the surgery, it was 35.1℃. Anesthesia lasted for 8 hours and 10 minutes, with the majority performed in the prone position. The estimated blood loss was 300 ml with a hemoglobin level of 10.2 g/dl without a transfusion. At the end of the anesthesia period, the residual neuromuscular block was reversed with 2.0 mg of neostigmine and 0.4 mg of glycopyrrolate and a train-of-four ratio of 1.0 was confirmed. The patient did not respond to verbal commands but breathed spontaneously with adequate tidal volume, and entropy was maintained over 95. Therefore, her condition was judged to be appropriate for extubation.
Upon arrival at the postanesthesia care unit (PACU), the patient still did not respond to verbal commands such as “open your eyes,” “what is your name,” and “squeeze my hand,” exhibiting only painful moaning with firmly closed eyes and mouth. Her condition remained unchanged for the next 3 hours during her stay in the PACU, and we could only surmise from her facial expressions and moaning that she was in pain. A blanket and warmer were applied to treat hypothermia (35.1℃) throughout her stay in the PACU, and her temperature reached 36.1°C just before the transfer to the ward. Hypoglycemia and electrolyte abnormalities were ruled out (blood glucose concentration, 11.3 mmol/L; serum sodium, potassium, calcium, magnesium, and chloride, 140, 4.0, 4.4, 1.1, and 106 mmol/L, respectively). Her pupillary reflex could not be checked because her eyes were tightly closed, and a detailed neurological examination could not be performed due to her non-cooperation. However, there was no evidence of a cerebrovascular accident (CVA). She was hemodynamically stable, with a blood pressure of 140–170/60–90 mmHg and an SpO2 of 97–98% on room air. The modified Aldrete’s score was 8 due to unresponsiveness. We decided to transfer her to the general ward and obtain a neurology consultation for further evaluation.
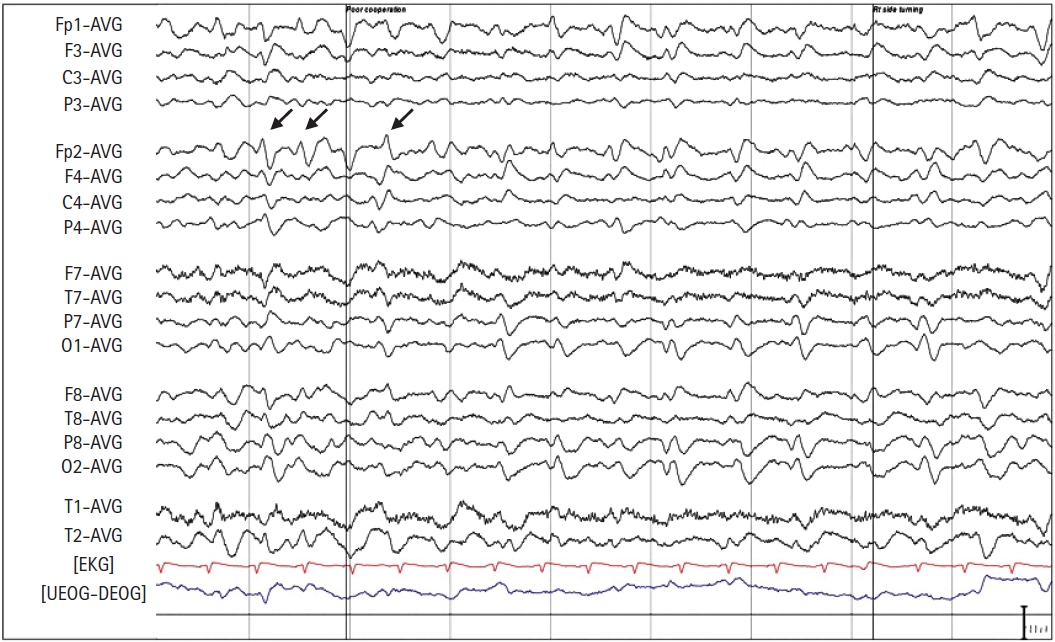
The day after the surgery, the patient was transferred to the neurology service for evaluation and workup of her mental status change and the possible CVA occurrence. The neurological examination by a specialist showed no abnormal findings suggestive of a CVA. She voluntarily moved all four extremities upon occasion and responded to painful stimuli but remained mute and unresponsive to verbal commands. However, at most times, she seemed awake and possibly aware of her surroundings. At postoperative 18 hours, magnetic resonance imaging (MRI) and EEG were performed to differentiate between an ischemic stroke and NCSE in relation to speech impairment or unresponsiveness after anesthesia. The MRI findings were normal; the EEG showed a large number of generalized slow waves, and periodic lateralized epileptiform discharge-like patterns appeared occasionally over the right hemisphere (Fig. 1). After ruling out CVA and confirming the EEG pattern, she was given an intravenous loading dose of 200 mg of lacosamide and maintained on an oral dose of 50 mg of lacosamide twice a day starting on postoperative day 1. Over the next two days, she gradually became more alert and followed verbal commands and started ambulation on the third postoperative day. Thereafter, she was maintained on lacosamide, and no further episodes indicating NCSE were reported during her two-month follow-up period.
Discussion
Decreased responsiveness or alertness after general anesthesia poses many difficulties to anesthesiologists. Although there is no established definition of delayed emergence even after long periods of anesthesia, patients usually restore their response to stimuli and consciousness within 1 hour after discontinuing anesthetics.
Several factors are attributed to the delayed recovery from anesthesia in the immediate postoperative period. First, pharmacologic factors should be excluded. The residual effects from various anesthetics such as volatile anesthetics, opioids, and neuromuscular blocking agents could be responsible for delayed emergence or unresponsiveness. In our case, complete recovery from neuromuscular blocking agents was confirmed with a neuromuscular transmission monitor. The patient received a total of 3 mg of remifentanil and desflurane with an end-tidal concentration of 4% for the maintenance of anesthesia, which lasted for almost 8 hours. Long anesthesia time could contribute to delayed emergence, but it was quite unusual that the residual anesthetic effects lasted so long, considering that remifentanil and desflurane are known for their short duration of action.
Next, metabolic causes, such as hypothermia, hypoglycemia, and electrolyte imbalance, should be considered for this circumstance. Upon arriving at the PACU, the patient’s temperature was 35.1℃, which gradually rose to 36.1℃. However, her condition remained unchanged despite a rise in body temperature. Moreover, the patient’s blood electrolyte concentrations were within the normal ranges, and hypoglycemia was not noted. After ruling out the usual causes of delayed emergence, neurologic events, which are relatively uncommon, but with serious sequelae, should also be considered as a possible etiology. The patient’s non-cooperation made it impossible to complete a neurologic examination in the immediate postoperative period. There was no lateral sign implying CVA. However, the subsequent MRI study and a complete neurologic examination by the neurologist confirmed that. If the patient were suspected of having a stroke, we would have called for a neurologist immediately. A surgical emergency, however, was excluded based on the benign physical exam, and the patient’s cardiovascular system was stable. Those non-specific findings made us hesitate to consult with the neurologist quickly.
NCSE is a condition with symptoms ranging from subtle muscle twitches to generalized coma and is difficult to diagnose unless an EEG shows the presence of seizure activity. The condition might be confused with delayed emergence or hypoactive delirium after anesthesia, especially in geriatric patients.
There is substantial variability in the reported incidence of NCSE, reflecting the lack of a universally accepted definition and the difficulty in diagnosis [6]. NCSE is likely to occur in any condition that causes brain tissue injury such as subarachnoid hemorrhage, traumatic brain injury, and intracranial surgery [7,8]. NCSE has been increasingly reported in other comatose patients in intensive care units [9]. The actual incidence would be supposed to be higher than reported, given that NCSE is easily missed. NCSE is particularly difficult to recognize in the postoperative period and often misinterpreted as residual anesthetic effects. NCSE is often a diagnosis of exclusion, and a high degree of suspicion is important. Jordan [10] suggested that NCSE should be suspected if consciousness is not restored for a long period after all types of surgery with a high risk of brain dysfunction. Old age and critical illness are known risk factors for NCSE development [11,12].
The prognosis of NCSE is highly related to an underlying disease, and when it is excluded, the prognosis may vary depending upon the presenting level of consciousness [13]. Newly developed status epilepticus in the elderly, a subtype of NCSE, is a morbid condition with mortality ranging up to 57% [1]. However, the prognosis in certain subsets of patients with de novo absence status is benign, such as in our patient who had no concomitant brain or systemic injury. But this does not mean that the treatment decision should be based solely on the expected outcome. Especially in postoperative patients, the risk of delayed ambulation and its associated potential complications, unnecessary diagnostic studies, and hospital costs should be considered. Although rare, delays in the diagnosis and initiation of treatment could have neurological sequelae [1,2].
With regard to treatment options, the response to initial treatment with benzodiazepines such as lorazepam is usually good but sometimes is delayed in elderly patients with de novo reactive status [2]. Devarajan et al. [3] reported that seizures slowly decreased 8 hours after initiating treatment and persisted for another 5 hours in the patient with newly developed NCSE in the immediate postoperative period. A follow-up EEG is not mandatory to confirm the therapeutic effect of anti-epilepsy medications, and the patient’s symptoms gradually recovered over two days in our case.
The presence of seizure activity in the EEG is essential for the diagnosis and treatment of NCSE; but EEG is not one of the routine diagnostic tests for the presence of delayed emergence in the PACU, and obtaining an emergency EEG after regular working hours and during weekends is unavailable in some institutions. In this case, the patient stayed in the PACU for about 3 hours starting at 5 PM, and it was not until around 6 PM that the patient’s condition raised our attention. Since we placed more possibility on hypoactive delirium and it was not regular working hours, the subsequent examination and referral were decided to be performed early the next day. However, as mentioned earlier, if circumstantial evidence highly suggestive of NCSE exists, a neurologic consultation should be sought as soon as possible for diagnosis and treatment.
Decreased consciousness can arise from serotonin syndrome and overlapping features with NCSE may lead to a misdiagnosis. Serotonin syndrome resulting from serotonin excess usually presents as a triad of neuromuscular hyperactivity, autonomic instability, and mental status changes, and the patient’s history reveals current exposure to a serotonergic agent [14]. The patient reported here had been prescribed 75/650 mg of oral tramadol/acetaminophen twice daily, 5/2.5 mg of oral oxycodone/naloxone twice daily, and 100 mg celecoxib twice daily for back pain. Fentanyl was also administered for pain control in the PACU. Opioids including tramadol, oxycodone, and fentanyl have been considered serotonergic agents associated with serotonin syndrome [15]. Fentanyl in the setting of other serotonergic opioids might have caused the serotonin syndrome in our patient. This hypothesis, however, is not supported by the patient’s symptoms as the patient did not show any autonomic instability including hyperthermia, hypertension, diaphoresis, or neuromuscular hyperactivity such as muscle rigidity, tremors, and bilateral Babinski signs. Initially, serotonin syndrome was ruled out based on the symptoms, and later NCSE was confirmed by EEG.
In conclusion, this case described de novo NCSE developments in an elderly woman after general anesthesia. This condition can be easily misdiagnosed, particularly in an unusual clinical setting such as the immediate postoperative period in the PACU and surgical locations other than the brain. The presence of NCSE after intracranial surgery or any other type of surgery should be considered in patients with an unexplained impairment of consciousness after anesthesia. Patients receiving an appropriate diagnostic approach and quick treatment are more likely to have a better prognosis.
Notes
Funding
None.
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Kyoung Ok Kim (Conceptualization; Writing – original draft)
Teakseon Lee (Data curation)
Taehoon Kim (Data curation)