Paravertebral block: anatomy and relevant safety issues
Article information
Abstract
Paravertebral block, especially thoracic paravertebral block, is an effective regional anesthetic technique that can provide significant analgesia for numerous surgical procedures, including breast surgery, pulmonary surgery, and herniorrhaphy. The technique, although straightforward, is not devoid of potential adverse effects. Proper anatomic knowledge and adequate technique may help decrease the risk of these effects. In this brief discourse, we discuss the anatomy and technical aspects of paravertebral blocks and emphasize the importance of appropriate needle manipulation in order to minimize the risk of complications. We propose that, when using a landmark-based approach, limiting medial and lateral needle orientation and implementing caudal (rather than cephalad) needle redirection may provide an extra margin of safety when performing this technique. Likewise, recognizing a target that is not in close proximity to the neurovascular bundle when using ultrasound guidance may be beneficial.
Introduction
The thoracic paravertebral space (PVS) is a wedge-shaped space, with its base facing the lateral sides of the vertebral bodies and intervertebral foramina, and the apex being continuous with the intercostal spaces. It is bound posteriorly by the superior costotransverse ligament (SCTL), anterolaterally by the pleura, medially by the vertebrae and intervertebral foramina, and superiorly and inferiorly by the ribs (Fig. 1). It is generally considered to end at L1 with no defined cranial border. Each segment of the PVS communicates superiorly and inferiorly over the rib head and neck and is sometimes compartmentalized into anterior and posterior sections by the endothoracic fascia. This space contains the branching spinal nerve, sympathetic nerve fibers, and intercostal vessels embedded in adipose tissue and is usually continuous over the thoracic levels. The orientation of the neurovascular bundle (NVB) changes from medial to lateral in the PVS, with the intercostal vessels and nerves arising anteromedially, but eventually lying directly beneath the rib between the internal and innermost intercostal muscles (Figs. 2 and 3).
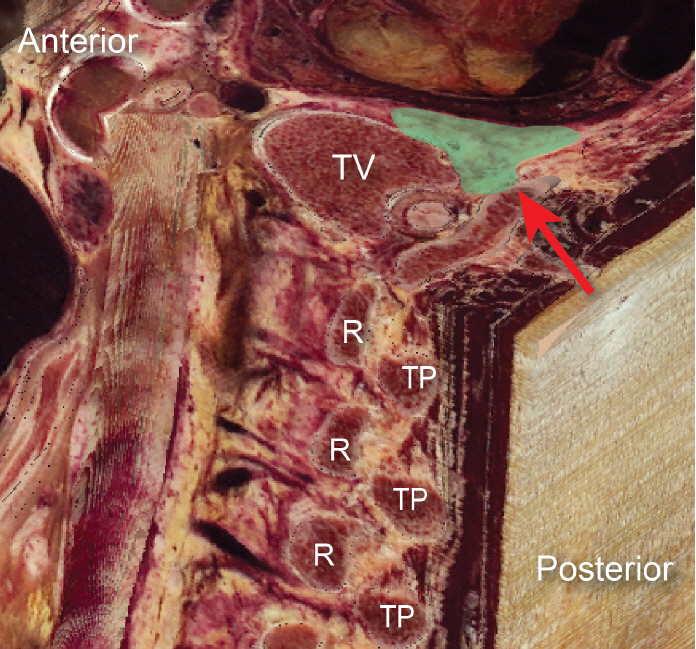
Composite parasagittal/transverse image of the thoracic spine. Green relief indicates paravertebral space, red arrowhead identifies superior costotransverse ligament. R: rib, TP: transverse process, TV: thoracic vertebra.

Paravertebral anatomy. (A) Landmarks of the paravertebral space (PVS) are shown in deep dissection and (B) with corresponding reconstructed computed tomography angiography using Anatomage system. A: thoracic transverse process, B: intercostal neurovascular bundle, C: partially dissected superior costotransverse ligament, D: pleura, red arrowhead: spinal nerve, PVS at tip of blue arrow: paravertebral space, R: rib, SP: spinous process, TP: transverse process. Notice the location of the neurovascular bundle immediately below the TP in the dissection.
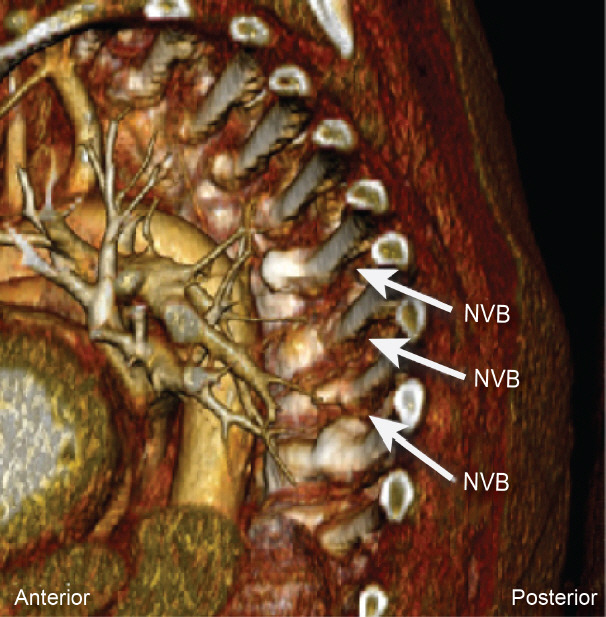
Reconstructed computed tomography angiography using Anatomage system showing the location of neurovascular bundle (NVB) relative to the rib and vertebral body. Note intercostal arteries and veins running along the inferior aspect of the ribs.
Proper identification of the space has traditionally been accomplished with landmarks using a predetermined distance lateral to the spinous process (SP) and/or loss of resistance. In some cases, this landmark technique has also been performed in combination with nerve stimulation, seeking an intercostal muscle twitch from intercostal nerve stimulation. More recently, ultrasound-guided (USG) paravertebral block (PVB) has become popular. Most approaches typically require identification of the transverse process (TP), and subsequently the SCTL (Fig. 4). While the landmark technique requires contact with the TP, other approaches, such as the lateral intercostal technique, do not require contact with this osseous structure [1].
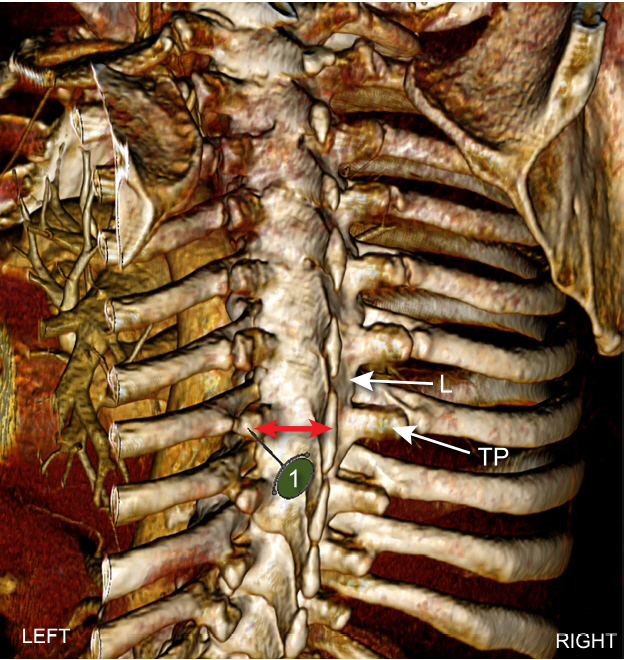
Computed tomography angiography reconstruction in a coronal plane using Anatomage system showing location of transverse process (TP) in relation to ribs and lamina (L). A needle insertion point 2.5–3.0 cm lateral to spinous process maximizes the likelihood that the needle (1) will contact TP and not rib or lamina. Red arrows denote mean distance of 2.5–3.0 cm as measured by software.
As an anatomic space, the PVS will accommodate local anesthetic that can spread into cephalad, caudal, intercostal (including the dorsal intercostal compartments), interpleural, epidural, and prevertebral spaces. Depending on the volume, it may stay at the level of injection or spread to additional intercostal spaces with a caudal preference [2]. Similarly, bilateral spread is more likely if a large volume of local anesthetic is injected at a single site, as opposed to multiple low-volume injections at several adjacent sites.
Injection in the PVS should be easy and without resistance, with the goal of generating unilateral sensory, motor, and sympathetic blockade, though the somatic and sympathetic blockade may be variable. Its rapid onset is generally attributed to the local anesthetic being deposited into a small compartment containing small-sized nerves without a substantial covering of fascia [3]. Successful PVB will result in loss of cold sensation at the associated dermatomes to which the block was applied.
Discussion
Although serious adverse effects associated with PVB are relatively rare, they can include, but are not limited to, pleural puncture, pneumothorax, vascular puncture, nerve injury (central and peripheral), organ damage, local anesthetic toxicity, reaction to adjunct medications, post-dural puncture headache, and aberrant spread of local anesthetic (central and peripheral) [1]. Block failure rate with a landmark-based technique has been estimated in the literature as 6% to 10% [4]; however, this figure may be much lower in the hands of experienced anesthesiologists. For example, at Mayo Clinic Florida our PVB block failure rate is less than 1% after performing more than 5000 blocks (unpublished raw data). The overall incidence of adverse effects is usually no more than 5%, with hypotension being the most common development (4.6%), followed by vascular puncture (3.8%), pleural puncture (1.1%), and pneumothorax (0.5%) [3–5]. Nevertheless, patients for whom PVB is considered should be carefully evaluated for applicability and risk stratification; rare and devastating adverse effects, including pulmonary hemorrhage and development of chronic pain and Brown-Séquard syndrome, have been reported [6,7]. Here we will review the technical details of the landmark-based and USG techniques and discuss the relevant safety points associated with each.
Landmark technique
With the patient in a seated position, the superior edge of the SP is identified at the desired level. The location of the TPs can differ depending on the desired vertebral level of blockade, and cadaveric analysis describes that thoracic TPs encountered during PVB placement tend to correlate with the spinous process of the vertebral body 1 level cephalad [8].
The needle insertion point is marked approximately 2.5 cm lateral from the midline. At this point, a 22-gauge Tuohy needle (B. Braun Medical, USA) is inserted perpendicular to the skin, with the goal of contacting the TP (Fig. 4). Distances from the midline can be variable, but a more lateral starting point may result in missing the TP, and a more medial orientation risks violation of the neuraxis. Likewise, relationships between the lateral point of insertion and the depth of the TP can be variable (3–5 cm), generally requiring deeper needle insertion in the cervical and lumbar areas, and shallower distances in the thoracic regions. In patients with difficult surface anatomy, ultrasound can be a useful adjunct to assess depth to this bony structure and distance from the midline [9].
Once the TP is contacted, generally at a depth of 2 to 5 cm in adults, the needle is withdrawn into the subcutaneous tissue, redirected in a caudal direction, and then slowly advanced with the purpose of entering the PVS at an approximate depth of 1.0 to 1.5 cm past the initial contact with the TP. The depth of the PVS has been estimated at 3 to 6 cm from the skin surface, and passage through the SCTL may be appreciated in the thoracic region as a loss of resistance or, if a blunt needle is being utilized, a small ‘pop’. If bony contact is not obtained at the above suggested needle depth, it is likely that the needle tip is between the TPs. The needle is then redirected in an arc cephalad and caudad until the TP is contacted. If the TP is still not contacted it is assumed the needle is too superficial. The needle is then advanced 1 cm deeper and the process repeated until the TP is contacted. Once the TP is contacted and the needle redirected caudad off the TP, we suggest that excessive caudal angulation of the needle (> 45 degrees) should be avoided. In our experience, this appears to be associated with an increased risk of failed nerve block and adverse effects.
Considerations to improve safety during landmark technique
In the thoracic region, a loss of resistance or a ‘pop’ is usually associated with traversing the SCTL, while in the lumbar region, this same phenomenon is not associated with the PVS and may be instead indicative of a violation of the psoas fascia. Loss of resistance when entering into the PVS can also be appreciated, but given its subjective nature, we suggest that using predetermined parameters (i.e., needle depth of no more than 1.0–1.5 cm past the original point of contact with the TP) can help avoid adverse effects. Using the TP as an initial contact location for PVB provides a good reference point prior to further needle manipulation, especially when considering the proximity of the NVB and pleura. As Fig. 5 shows, even with advancement of the needle just 1.5 cm past the point of contact with the TP, the needle tip can be in close proximity to the pleura (with either a cephalad or caudal redirection). While both caudal and cephalad redirections of the needle after initial contact with the TP have been advocated, it is our opinion that, when using a landmark-based approach, consistently employing a caudal needle redirection to the PVS and minimizing medial and lateral deviation will lead to fewer adverse effects as the following four situations demonstrate:

Relationship between lung and potential needle placement. (A) Anatomic reconstruction of lung windows (B) with reconstructed computed tomography of thoracic spine in sagittal plane. Numbers indicate 1: needle tip location with initial placement, 2: cephalad reorientation & 1.5 cm advancement, and 3: caudal reorientation & 1.5 cm advancement. Note proximity of the needle tips to the lung parenchyma with either direction.
1. Cephalad redirection of the needle after contact with the TP. Once the TP is located (Fig. 6A-1 and 6B-1), and the needle redirected, cephalad, the NVB, pleura, and lung lie directly within the needle path and are at risk of violation (Fig. 6A-2 and 6B-2, 4, and 5).

Approach to the paravertebral space. (A) Reconstructed computed tomography of the thoracic spine showing potential needle trajectory in sagittal plane, (B) gross dissection of paraspinal area in coronal plane. Item 1 shows place of initial contact with transverse process (TP). Item 2 shows that a cephalad approach after walking off the TP would result in close anatomic proximity to the neurovascular bundle (NVB). Item 3 shows that a caudal approach after walking off the TP results in a protective angle away from NVB. Item 4 suggests that a medial redirection of the needle risks neuraxial violation. Item 5 suggests that a lateral redirection of the needle risks pleural violation.
2. Caudal redirection of the needle after contact with the TP. Placement of the needle tip on the TP with subsequent caudal redirection places the needle in a relatively avascular non-neural location and may be shielded by the TP (Fig. 6A-3 and 6B-3).
3. Medial redirection or contact with the lamina. If the initial needle placement is too medial on the TP, or on the lamina, subtle needle redirection can help provide a more accurate approximation of the bony anatomy. However, advancement towards the PVS should not be directed cephalad or medial as it approximates placement of a thoracic epidural, with the risk of neuraxial puncture (Fig. 6B-4). Alternatively, caudal redirection should again help limit neurovascular injury: if the needle tip is potentially on the lamina, caudal redirection helps take advantage of the natural protective angle of the thoracic vertebra. Consistent contact with bone may be due to continued contact with the lamina, necessitating reassessment of the initial insertion site.
4. Lateral redirection or contact with the rib. If the needle is initially placed on the lateral portion of the TP and directed cephalad and lateral, the NVB or pleura may be trespassed (Fig. 6B-5). However, directing the needle tip in a caudal direction will aid in bypassing the NVB. Likewise, if the rib is first contacted, cephalad redirection risks placing the needle in the NVB, pleura, or lung, while caudal redirection will identify the inferiorly located TP (i.e., more shallow bony contact as the TP is more superficial than the rib). The initial contact point should then be moved to the TP.
When an attempt to enter the PVS is performed as described, maintaining a caudal needle direction while observing the above parameters may considerably decrease risk of inadvertent pleural, vascular, or neural puncture, regardless of which structure is first contacted. Of note, the rates of vascular injury and pleural puncture quoted by Lonnqvist et al. [4] reflect a caudal-to-cephalad needle redirection during landmark technique. In contrast, with the use of a cephalad-to-caudal needle redirection technique at Mayo Clinic Florida, the rates of these two complications are less than 1% at that institution, respectively [unpublished raw data]. Additionally, excessive angulation of the needle during PVB placement should be avoided, as steep angulation can bypass critical bony structures, lead to inappropriate needle endpoints, and result in block failure or adverse events.
Ultrasound-guided technique
There are several USG techniques to the PVS that generally have a high success rate with few adverse effects. Ultrasound can be used to easily identify key landmarks and needle position. Care must be taken to properly visualize the entire needle, avoid neuraxial adverse effects, and appreciate the SCTL and anterior displacement of the pleura in the subset of USG techniques that require their identification. Theoretically, direct visualization of the needle should decrease risk of adverse effects, while simultaneously confirming proper local anesthetic placement with anterior displacement of the pleura.
Generally, the ultrasound probe is positioned in a transverse (Fig. 7A) or sagittal (Fig. 7B) orientation, though modifications to these approaches have been suggested [10]. The type of approach dictates which landmarks are identified. An in-depth analysis is described by Krediet and colleagues [10]. Here, we briefly list the salient characteristics of these approaches.

Ultrasound views of paravertebral space (PVS). (A) Transverse ultrasound view of the PVS. (B) Sagittal ultrasound view of the PVS. EIM: external intercostal muscle, IIM: internal intercostal membrane, TP: transverse process, PSM: paraspinal muscle.
1. Transverse USG probe orientation. With the ultrasound probe oriented transversely, key anatomic landmarks vary depending on the approach to the rib [1], TP [11–14], and inferior articular process [9] being used; parietal pleura, visceral pleura, and internal intercostal membrane may also be seen. Most approaches aim to place the tip of the needle between the internal and innermost intercostal muscles with a lateral-to-medial needle pathway and are performed in plane, though an out-of-plane medial-to-lateral approach has also been described [10].
2. Sagittal probe orientation. With the ultrasound probe oriented in the sagittal plane, the rib can be used as a lateral limit for transverse probe movement, and the TP as a medial limit [14,15]. With this probe positioning, the PVS will be visualized immediately caudal and anterior to the TP. The needle trajectory has been classically described as caudal-cranial or caudal-lateral/cranial-medial when in plane, and caudal-cranial out of plane.
Considerations to improve safety during ultrasound-guided technique
Both cadaveric and in vivo studies have shown that USG approaches can result in adequate spread of injectate within the PVS [10]. Pleural displacement can be a reliable visual end point for successful deposition of local anesthetic. While the use of ultrasound guidance will result in the ability to directly visualize the advancement of the needle during the block, the inherent risks of neuraxial violation when directing a needle lateral to medial, and the inability to visualize the entire needle in the out of plane approach, should always be taken into consideration. The use of ultrasound visualization can greatly aid in identifying (and thus avoiding) the NVB. With proper identification of these vascular structures, the block needle can be manipulated in a fashion that minimizes the risk of neural or vascular injury. Depending on the ultrasound approach being utilized, this needle movement may very well involve either a caudal-to-cranial (Fig. 8A) or anteromedial (Fig. 8B) redirection. Thus, when using ultrasound guidance, the direction of needle orientation becomes less important than the final endpoint of the needle as long as both needle tip and neurovascular structures are well identified. Under ultrasound guidance one should aim to manipulate the needle towards the caudal area of the PVB space and avoid approaching the cephalad area of the PVS, thus potentially minimizing the risk of needle contact with the NVB.

Needle manipulation under ultrasound. (A) Illustration of possible needle manipulation during sagittal-plane ultrasound-guided (USG) paravertebral block (PVB). Note that unlike landmark-based technique, the needle may be redirected cephalad safely without risking injury to the neurovascular bundle. Used with permission of Mayo Foundation for Medical Education and Research, all rights reserved. (B) Illustration of possible needle orientation during transverse-plane USG-PVB. Note that with proper visualization of visceral and parietal pleura, the needle may be directed medially safely. Used with permission of Mayo Foundation for Medical Education and Research, all rights reserved.
Ultrasound-guided versus landmark technique
While ultrasound imaging is an invaluable tool in various regional anesthesia techniques, evidence regarding superiority of this technique compared to the landmark-based approach in PVB is mixed. A retrospective review by Saran et al. [16] found no difference in block efficacy, pain scores, opioid use, or complications between the two techniques. In contrast, a prospective randomized controlled trial among breast surgery patients suggested greater PVB success for USG techniques [17]. Perhaps not surprisingly, USG lateral-to-medial approaches to the PVS have been associated with higher incidences of epidural spread, believed to be due to needle direction toward the neural foramina and neuraxis, as compared to the landmark technique [5]. Conversely, the landmark technique takes advantage of the greatest anterior-posterior dimension of the PVS (i.e., medial vs. lateral location) and does not require medial-to-lateral or lateral-to-medial needle direction, thereby limiting the dangers associated with these trajectories. Regardless of theoretical or actual advantages of using ultrasound for Paravertebral blockade, anatomic knowledge is still the most important factor in maximizing block success and safety. Further studies comparing the two techniques are necessary; in some patient populations the landmark technique can still play an important role.
Conclusion
Paravertebral blockade is an excellent regional anesthetic technique for primary or adjunct anesthesia and analgesia. Appropriate patient selection, anatomic knowledge, and proper technique are essential to patient safety. In landmark techniques accessing the TP, redirecting the needle caudally after contacting the TP may improve the safety of this block. Among USG techniques, actively manipulating the needle to avoid the NVB may similarly improve safety.
Acknowledgements
Editing, proofreading, and reference verification were provided by Scientific Publications, Mayo Clinic.
Notes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Alberto E Ardon (Investigation; Writing–original draft; Writing–review & editing)
Justin Lee (Conceptualization; Investigation; Writing–original draft; Writing–review & editing)
Carlo D. Franco (Investigation; Methodology; Writing–review & editing)
Kevin T. Riutort (Investigation; Methodology; Writing–review & editing)
Roy A. Greengrass (Conceptualization; Investigation; Writing–original draft; Writing–review & editing)
