Transcatheter tricuspid valve-in valve replacement-hope for the forgotten valve!
Article information
The tricuspid valve (TV) has been long been referred to as the ‘forgotten valve,’ though recently, understanding of the long-term consequences of untreated tricuspid pathology has improved.
The 2014 American Heart Association/American College of Cardiology guidelines for the Management of Patients with Valvular Heart Disease list Class I evidence for TV repair/replacement in patients with severe tricuspid regurgitation (TR) or stenosis undergoing left-sided valve surgery [1].
However, bioprosthetic valves undergo degeneration over time. Re-operative surgical valve replacement is the standard of care but can be associated with significant operative mortality and morbidity, ranging from 13% to 37% [2].
Transcatheter tricuspid valve-in-valve (TVIV) implantation has emerged as an attractive alternative to surgical valve replacement in bioprosthetic valve dysfunction in patients at prohibitive surgical risk.
We present a patient who successfully underwent transcatheter TVIV implantation as a salvage treatment option for bioprosthetic failure. Written consent was obtained. A 38-year-old male was admitted with symptoms of significant scrotal edema causing severe pain and impairing ambulation, shortness of breath, paroxysmal nocturnal dyspnea, orthopnea, and ascites. Past medical history included human immunodeficiency virus infection on highly active antiretroviral therapy, hypertension, pulmonary artery pseudoaneurysms, and substance abuse complicated by multimicrobial TV endocarditis. He had undergone two previous open TV surgeries (one for repair, one for replacement) with poor recovery following sternotomy, bioprosthetic valve endocarditis subsequently treated, and septic pulmonary emboli. He was diagnosed with severe bioprosthetic TV regurgitation and co-existing TV stenosis. A pre-operative transesophageal echocardiogram (TEE) revealed a TV mean pressure gradient of 17 mmHg and no evidence of vegetations on the valve. Due to his comorbidities and past surgical history, he was deemed a prohibitive-risk surgical candidate. After multidisciplinary discussion, a decision was made to perform a transfemoral transcatheter TVIV replacement.
After placement of a pre-induction arterial line, general anesthesia (GA) was induced and a central venous catheter and TEE probe placed post-induction. Pre-procedural TEE revealed severe TR and stenosis, with mean pressure gradient of 14 mmHg. Femoral artery access was secured by the surgical team to facilitate emergent cardiopulmonary bypass, if needed. A Swan-Ganz catheter inserted from the femoral vein into the right ventricle (RV), was exchanged in a series of steps and a 29-mm Edwards Sapien 3 valve successfully deployed (Fig. 1) within the bioprosthetic TV. Hemodynamic stability improved immediately. TEE showed significant reduction in the TV mean pressure gradient from 14 to 1 mmHg with no residual TR. The procedure was well tolerated, and the patient extubated in the operating room with no adverse postoperative events.
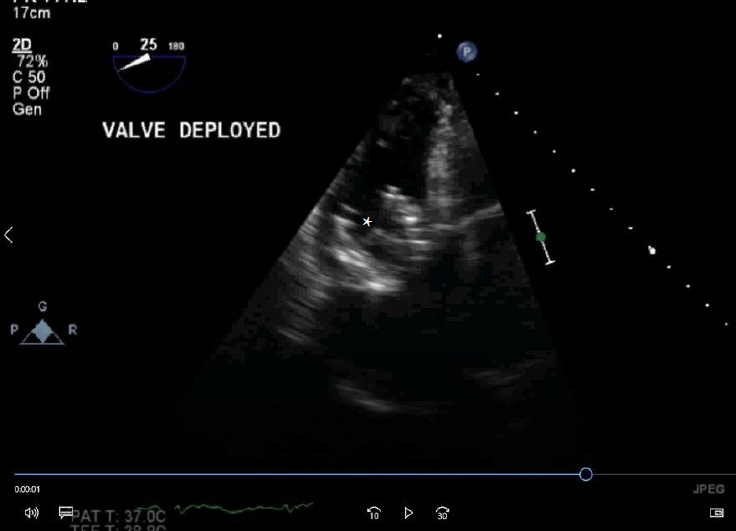
Post implantation. Deep transgastric view of the right ventricle showing the deployed Sapien 3 valve (*) in tricuspid position.
While 300 cases have been reported over the last decade, TVIV is still uncommon. Successful anesthetic management requires assessment and optimization of comorbidities common in these patients, such as right heart failure, liver and renal dysfunction, and debilitation. It is also important to understand procedure-related special considerations.
Although it is feasible to perform these procedures under sedation, GA is preferred when intraoperative TEE is used. Invasive monitoring consists of arterial line and central venous catheter insertion due to potential for hemodynamic instability and necessity for vasopressor administration. Anticoagulation is typically achieved with 100 units/kg of heparin to achieve an activated clotting time of > 250 s, after vascular access is obtained by the surgeons [3]. Since there is a risk of a right ventricular pacing lead being jailed between the two prostheses or damaged, pacing during valve deployment may be induced by an electrode placed in the right atrium, coronary sinus, or left ventricle. The valve may also be deployed without pacing—as in our patient—due to the low flow velocity in the right ventricular outflow tract, as compared to the left side. Adenosine has also been used during valve deployment. These patients are usually extubated at the end of the case.
Several major anatomical challenges exist in transcatheter TV therapy as compared to other valves [4]. The tricuspid annulus is large, non-planar, and elliptical in shape. This, coupled with the angulation of the tricuspid annulus relative to the superior and inferior vena cava, can preclude optimal alignment of the delivery system. The annulus is proximal to many critical structures—the right coronary artery, coronary sinus, atrioventricular node, and Bundle of His—which may be damaged during deployment. The thin right ventricular free wall is also vulnerable to injury. Trabeculations and muscle bands within the RV can impede device positioning and equipment retrieval. Preoperative echocardiography is important not only to assess the severity and etiology of bioprosthetic valve failure but also to identify contraindications such as endocarditis. Evaluation of prosthesis size is important and determined via an integrated approach informed by the manufacturer’s reported internal diameter, mean diameter as measured by computed tomography, three-dimensional (3D) TEE, and fluoroscopy [5]. The angulation of the valve annulus in relation to the access route is also assessed. Intraoperatively visualization is provided by 2D and 3D TEE and fluoroscopy. TEE can confirm transcatheter valve position before deployment, assess for post-implantation complications, and evaluate function. Functional evaluation includes confirmation of correct position, annular stability, leaflet mobility, assessment of pressure gradients and valve area, as well as presence and severity of any intravalvular or paravalvular regurgitation. TEE provides early detection of malposition, embolization events, and pericardial effusion.
To conclude, rapidly evolving advancements in the percutaneous treatment of structural heart disease coupled with improved understanding of the long-term consequences of untreated tricuspid pathology have led to the emergence of transcatheter TV replacement as a promising option for management of high-risk patients with failed surgical bioprostheses. It is important for the anesthesiologist to understand the special considerations involved with transcatheter TVIV.
Notes
No potential conflict of interest relevant to this article was reported.
Author Contributions
Seema P. Deshpande (Conceptualization; Writing – original draft; Writing – review & editing)
Susan Sankova (Writing – original draft; Writing – review & editing)
Nicolas Dorsey (Writing – original draft; Writing – review & editing)
Murtaza Y. Dawood (Writing – original draft; Writing – review & editing)
Kenichi Tanaka (Writing – original draft)
