|
 |
| Korean J Anesthesiol > Volume 73(6); 2020 > Article |
|
Abstract
Background
The effects of anesthetic techniques on postdural puncture backache (PDPB) have not been specifically evaluated. The purpose of this study was to compare the incidence and severity of PDPB between median and paramedian techniques.
Methods
Patients were randomized to receive spinal anesthesia by either a median (Group M, n = 50) or paramedian (Group P, n = 50) approach.We recorded each patient’s personal number of puncture attempts, surgical position, and operation duration. We investigated the incidence and intensity of back pain 1 day, 1 week, and 1, 2, and 3 months postoperatively.
Results
The overall incidence of PDPB was higher in the Group M (18/50, 36%) than in the Group P (8/50, 16%) (P = 0.023). Twenty-four hours after surgery, 8 patients in Group M and 6 patients in Group P complained of back pain. Seven days after the surgery, 16 patients in the Group M and 5 patients in the Group P complained of pain (P = 0.007). After 1 month, 5 patients in the Group M and 1 patient in the Group P complained of pain. Only one patient in each group complained of pain after 3 months. No significant differences were noted in NRSs between the groups during study period.
Spinal anesthesia is the most common regional anesthesia employed in many types of surgery, including urogenital organ surgery, cesarian section, and lower limb surgery. Postdural puncture backache (PDPB), which is characterized by continuous pain around the site of spinal puncture without any radicular pain, is a common complication after spinal anesthesia [1]. The reported incidence of PDPB ranges from 2% to 29% [2,3]. Excessive stretching of spinal ligaments by paraspinal muscular relaxation and/or localized tissue trauma are proposed as pathophysiological factors for PDPB [1].
Spinal anesthesia is performed by injecting local anesthetics into the subarachnoid space of the lumbar region. The subarachnoid space is accessible through median and paramedian approaches in either a sitting or a lateral position [4]. In the median technique, the needle is inserted below the lower edge of the spinous process of the selected upper vertebrae and passes through the supraspinous ligament, interspinous ligament, ligamentum flavum, and epidural space, piercing the dura mater. In the paramedian technique, the needle is inserted 1 cm lateral and 1 cm caudal to the caudal edge of the most superior spinous process in the sagittal plane. In this technique, the interspinous and supraspinous ligaments are not penetrated, and the ligamentum flavum is the first structure the needle encounters.
Previous studies suggested that patients are more likely to experience PDPB after spinal anesthesia using a large-bore spinal needle due to the increased degree of tissue injury [5]. The effect of the anesthetic technique on PDPB has not been specifically investigated. The median approach technique may aggravate stretching of the spinal ligaments, resulting in increased rates of PDPB. We postulated that avoiding penetration of the supraspinous and interspinous ligaments may decrease spinal ligament stretch and reduce the incidence of PDPB. The purpose of this study was to compare the incidence and severity of PDPB following the performance of the median and paramedian techniques.
This study was conducted under the approval of the Institutional Review Board of Gwangju Christian Hospital in 2017 (KCHIRB-M-2017-045).All patients signed an informed consent form before undergoing surgery. Data were collected from March 2017 to August 2017. One hundred and twenty-four patients with the American Society of Anesthesiologists physical status classification I–II, who were between the ages of 20–70 years and were scheduled for elective surgery under spinal anesthesia, were enrolled. The types of surgery included urological, orthopedic, gynecological, and general surgeries. Patients with pre-existing low back pain and those who were unable to converse or ambulate after surgery were excluded. Patients with traumatic deformity of the spine or congenital abnormalities of the lumbar spine, as well as those with contraindications for spinal anesthesia, were also excluded.Patients were classified into either the median (Group M) or the paramedian group (Group P) by computer-generated randomization. Patients were excluded from the analysis when two or more punctures were attempted or when spinal anesthesia failed.
In the operating room, the blood pressure, heart rate, electrocardiogram, peripheral oxygen saturation, and respiratory rate of all patients were monitored. After infiltration of the puncture site with 5 ml of 2% lidocaine (Lidocaine HCl Hydrate Inj. 2%, Daihan), spinal anesthesia was performed with 0.5% hyperbaric bupivacaine (Bupivacaine HCl Heavy Injection 0.5%, Hana Pharm Co., Korea) at the L3–4 or L4–5 intervertebral space. All spinal anesthesia was performed in the lateral position by the same practitioner (Dr. Lee) using a 25-gauge (G) pencil point needle (Pencan, B. Braun, Malaysia). All patients were equipped with an IV patient-controlled analgesia (PCA) device for 48 hours for postoperative pain control. The total volume of analgesic solution was 100 ml, with a combination of 500 μg fentanyl (Fentanyl citrate, Hana Pharm Co., Korea) and 6 g propacetamol (Denogan, Youngjin Pharm Co., Korea). The IV-PCA was infused at 2 ml/h, and the bolus dose was 2 ml (30 minutes lock-out times) in all patients.
We recorded the patients’ personal data, including sex, age, weight, medical history, method of approach, puncture site, bupivacaine capacity, number of puncture attempts, sensory nerve block height, surgical position, operation time, and rescue analgesics (diclofenac, ketorolac, tramadol, and meperidine) added to IV-PCA. The doses of tramadol and meperidine used on the nursing floor were converted into equivalent morphine doses according to the Opioid Conversion Ratios-Guide to Practice 2010. We designed the questionnaire specifically to evaluate back pain and not transient neurological symptoms (e.g., unilateral or bilateral pain or radicular pain in the buttock, thigh, calves, or legs, as defined by Hampl and colleagues, Appendix 1) [6]. Twenty-four hours after operation, we interviewed patients and assessed their level of low back pain. If patients had back pain, we inquired about the characteristics, aggravating factors, and degree of pain using a numeric rating scale (NRS, NRS-11) [7]. We interviewed the patients and asked the same questions over the telephone after 7 days, 1 month, 2 months, and 3 months. The interviewer was not aware of the injection approach. Patient satisfaction was assessed at the end of the survey by asking whether the patient would choose to receive spinal anesthesia again (Appendix 1).
Previous studies indicated that the incidence of PDPB ranges from 2% to 29% [5,8,9]. To detect a difference of 0.3 between the two groups, sample size calculation suggested 44 subjects per group for a two-group 0.05 one-sided t-test with 80% power. Since we planned to use a non-parametric test, 50 subjects in each group were considered adequate for the present study (12% more than the calculated sample size). Data are presented as mean ± SD for continuous data and frequencies for categorical data. Statistical analyses were performed using SPSS ver. 18.0 software (SPSS, Inc., USA). Independent-samples t-tests for quantitative data and Chi-square test for qualitative data were applied. P values less than 0.05 were considered statistically significant.
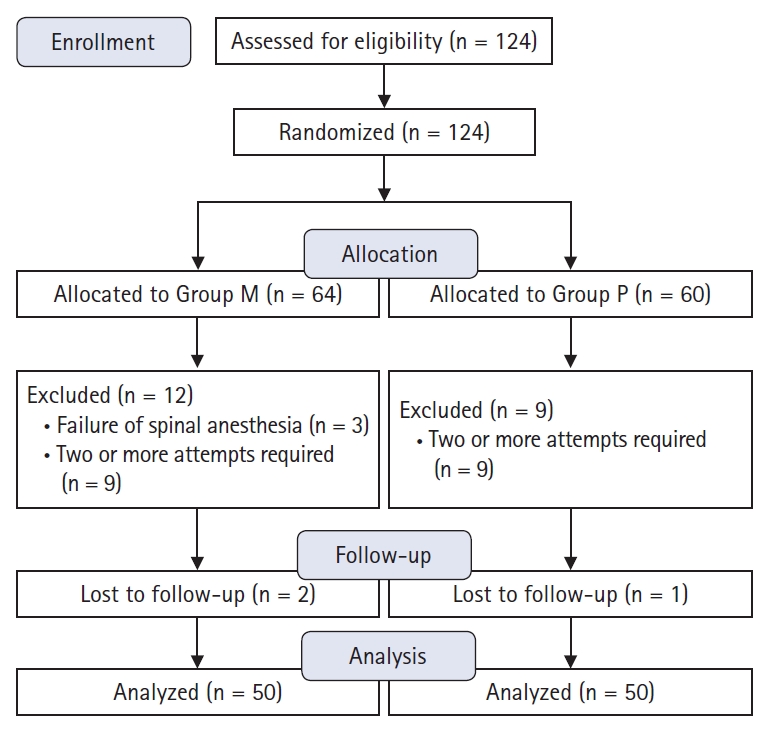
One hundred and twenty-four patients were enrolled in this study. Three patients in Group M were excluded due to failure of spinal anesthesia. Nine patients in Group M and 9 in Group P were excluded from the analysis because two or more attempts were required for successful needle placement. Two patients in Group M and 1 in Group P were lost to follow-up. Thus, a total of 100 patients were included in this study (Fig. 1). Demographic data are shown in Table 1 and did not differ between groups. Surgery type, operative time, surgical position, and bed resting time showed no evidence of differences between the groups (Table 2). In total, 18 (36%) patients in the Group M and 19 (38%) patients in the Group P received additional analgesia. Five patients in the Group M and 6 patients in the Group P received a single dose of diclofenac (75 mg). Nine patients in the Group M and 7 patients in the Group P received a single dose of ketorolac (30 mg). Six (12%) patients in the Group M and 8 patients (16%) in the the Group P received additional analgesia with tramadol and meperidine. Consumption of tramadol and meperidine was similar in both groups (0.8 ± 0.2 vs. 0.7 ± 0.1 mg by opioid conversion ratio) (Table 3).
During the 3-month follow-up period, 26 participants reported back pain (newly occurring) at least once. In the questionnaire administered 24 hours after surgery, 8 patients in the Group M and 6 in the Group P complained of back pain (P = 0.569); the NRS scores were 3.5 and 4.1, respectively (P = 0.828). Seven days after the surgery, 16 patients in the Group M and 5 in the Group P complained of pain (P = 0.007), with NRS scores of 3.9 vs. 3.8, respectively. After 1 month, 5 patients in the Group M and 1 in the Group P complained of pain (P = 0.095). The NRS score was 3.0 in both groups. One patient in the Group M and 1 in the Group P reported pain continuously for 3 months (Table 4, Fig. 2). Interestingly, both patients were operated upon in the lithotomy position.
Overall, 46/50 (92%) of patients in the Group M and 48/50 (96%) of patients in the Group P were satisfied with the spinal anesthesia.
We found that the overall incidence of PDPB was higher in the Group M than in the Group P, and the median approach technique was more frequently associated with PDPB 7 days postoperatively. Additionally, the pain intensity showed no evidence of differences between groups during the entire survey period, and PDPB lasting over 3 months was rare regardless of the technique.
One of the most common complications of spinal anesthesia is low back pain. Rhee et al. [10] studied patient dissatisfaction after spinal anesthesia and found that 54/1,191 (4%) patients were not satisfied. Twenty-nine percent of these dissatisfied patients identified back pain as the reason for their dissatisfaction. The risk factors for PDPB are as follows: preexisting back pain, immobilization of the spine for > 2.5 hours, lithotomy position during surgery, body mass index > 32 kg/m2, and multiple attempts made for needle placement [5,8,11–14]. The mechanisms which initiate PDPB include injury to the ligaments, fascia, or bone, with localized inflammation [13,15,16]. Potential exacerbating mechanisms include immobilization of the spine, relaxation of the paraspinal muscles due to spinal anesthesia, flattening of the normal lumbar convexity curve, and stretching and/or straining of the paraspinal ligaments and facet joints, especially in the lithotomy position [11,12].
PDPB is characterized by mild intensity, local tenderness at the site of injection, and responsiveness to oral anti-inflammatory drugs or spontaneous resolution [9]. A recent study reported that the incidence of PDPB decreased from 29% 1 day after spinal anesthesia to 5% 4 weeks after spinal anesthesia, and the intensity of pain also diminished over time [5]. The findings of the present study were consistent with those of previous studies. Two patients (1 patient in each group) complained of back pain that persisted for over 3 months.
Many studies have reported no effect of spinal needle parameters (needle type and size, and the use of an introducer) on PDPB [17–20]. On the other hand, a randomized study comparing the incidence and duration of back pain after spinal (24 G Sprotte spinal needle) and epidural (18 G Touhy epidural needle) anesthesia noted that the incidence of back pain was significantly higher on postoperative days 1, 2, and 3 after epidural anesthesia [21]. Additionally, a recent review article examining back pain and neuraxial anesthesia concluded that the incidence of back pain was higher after epidural anesthesia compared to spinal anesthesia [1]. Factors which may be responsible for the increased incidence of PDPB in the epidural group are the needle size and/or tip design. This result may suggest that greater degrees of penetration result in more back pain.
The majority of studies that compared different needle insertion techniques have reported that the incidence of PDPB is significantly lower in the Group P compared to the Group M [22–24]. In a recent randomized control study by Singh et al. [24], the incidence of back pain in the Group P and Group M was 2% and 10%, respectively. However, these studies have a few limitations; they focused on postdural puncture headache rather than back pain. They also lacked detailed exclusion criteria such as pre-existing back pain, patient position, immobilization time, and multiple needle placement trials. In addition, studies examining the occurrence of back pain after spinal or epidural anesthesia frequently fail to describe the characteristics of the back pain. Most studies did not comment on the characteristics of the back pain, and only a few studies noted the occurrence of radicular pain in the buttock and/or lower extremities [12,16].
In our study, the overall incidence of PDPB was significantly higher in the Group M compared to the Group P. At some time points, the incidence of back pain was higher in the Group M to a non-significant degree, and pain intensity was not different in both groups, except on day 7. Our results indicate that the median approach for spinal anesthesia may be a risk factor for PDPB in the early postoperative period, but is not correlated with long-term and/or chronic back pain. Because all patients received an IV-PCA device after surgery in our study, it is possible that back pain was underreported at the 24-hour time point. Despite the potential problem it might cause during data analysis, we equipped all patients with an IV-PCA device for ethical reasons.
The strengths of our study include the exclusion of patients with pre-existing back pain. Schwabe and Hopf [8] concluded that back pain after spinal anesthesia is almost exclusively associated with pre-existing back pain. Furthermore, a recent review article evaluating back pain and neuraxial anesthesia noted that pre-existing back pain is a risk factor for persistent back pain after neuraxial anesthesia [1]. We excluded patients with pre-existing back pain because our main goal was to evaluate newly occurring back pain following spinal anesthesia. In addition, our questionnaire, which has previously been used to evaluate back pain after epidural anesthesia, was designed specifically to evaluate PDPB and not transient neurological symptoms [14]. We also excluded results obtained from patients with multiple spinal needle insertions.
Our study has several limitations. First, we did not monitor the use of postoperative supplemental analgesics, such as non-steroidal anti-inflammatory drugs, due to ethical constraints. Second, we did not exclude patients who underwent surgery in the lithotomy position. Finally, although we found no significant difference in the incidence of PDPB between the two groups, definitive conclusions could not be drawn due to the small sample size.
We conclude that the paramedian approach for spinal anesthesia reduces the incidence of PDPB in the early postoperative period. Future studies involving a more detailed description of patients’ symptoms and an appropriate physical examination will help define the precise nature of the back pain and also assist in determining the appropriate treatment for such pain.
Fig. 2.
Total incidence of back pain was significantly higher in the median group. During the time course, the median group showed significantly higher incidence of back pain on day 7. Group M: median group, Group P: paramedian group. *Statistically significant with P < 0.05.
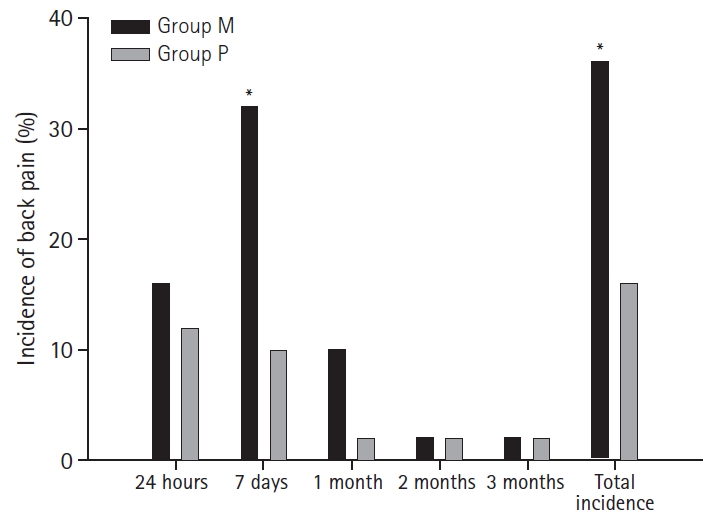
Table 1.
Demographic Data
Table 2.
Operative Information
Table 3.
Additional Use of Analgesics
| Group M (n = 50) | Group P (n = 50) | P value | |
|---|---|---|---|
| NSAIDs | |||
| Diclofenac | 5 (10) | 6 (12) | 0.677 |
| Ketorolac | 9 (18) | 7 (14) | 0.550 |
| Opioids | |||
| Tramadol and/or meperidine | 6 (12) | 8 (16) | 0.569 |
| Cumulative morphine (mg)* | 0.8 ± 0.2 mg | 0.7 ± 0.1 mg | 0.157 |
Values are presented as mean ± SD or number of patients (%). Group M: median group, Group P: paramedian group. NSAIDs: non-steroidal anti-inflammatory drugs. P values less than 0.05 were considered statistically significant.
* Opioid Conversion Ratios-Guide to Practice 2010. Available from https://swarh2.com.au/assets/A/4404/5e7e89de2ffbc6fd5cd11e38b7a85d53/OpioidConversion2010Final(2).pdf
Table 4.
Incidence and Severity of Pain
References
1. Benzon HT, Asher YG, Hartrick CT. Back pain and neuraxial anesthesia. Anesth Analg 2016; 122: 2047-58.


2. Cotev S, Robin GC, Davidson JT. Back pain after epidural analgesia. Anesth Analg 1967; 4: 259-63.


3. Brattebø G, Wisborg T, Rodt SA, Røste I. Is the pencil point spinal needle a better choice in younger patients? A comparison of 24G Sprotte with 27G Quincke needles in an unselected group of general surgical patients below 46 years of age. Acta Anaesthesiol Scand 1995; 39: 535-8.


4. Mosaffa F, Karimi KM, Khoshnevis SH. Comparison the incidence of PDPH after median and paramedian spinal anesthesia technique in patients undergoing orthopedic surgery. Anesthesiol Pain 2010; 1: 23-8.

5. Tekgül ZT, Pektaş S, Turan M, Karaman Y, Çakmak M, Gönüllü M. Acute back pain following surgery under spinal anesthesia. Pain Pract 2015; 15: 706-11.


6. Hampl KF, Schneider MC, Ummenhofer W, Drewe J. Transient neurologic symptoms after spinal anesthesia. Anesth Analg 1995; 81: 1148-53.


7. Hartrick CT, Kovan JP, Shapiro S. The numeric rating scale for clinical pain measurement: a ratio measure? Pain Pract 2003; 3: 310-6.


8. Schwabe K, Hopf HB. Persistent back pain after spinal anaesthesia in the non-obstetric setting: incidence and predisposing factors. Br J Anaesth 2001; 86: 535-9.



9. Pan PH, Fragneto R, Moore C, Ross V. Incidence of postdural puncture headache and backache, and success rate of dural puncture: comparison of two spinal needle designs. South Med J 2004; 97: 359-63.


10. Rhee WJ, Chung CJ, Lim YH, Lee KH, Lee SC. Factors in patient dissatisfaction and refusal regarding spinal anesthesia. Korean J Anesthesiol 2010; 59: 260-4.




12. Urbach KF, Lee WR, Sheely LL, Lang FL, Sharp RP. Spinal or general anesthesia for inguinal hernia repair? A comparison of certain complications in a controlled series. JAMA 1964; 190: 25-9.


13. Hakim SM, Narouze S, Shaker NN, Mahran MA. Risk factors for new-onset persistent low-back pain following nonobstetric surgery performed with epidural anesthesia. Reg Anesth Pain Med 2012; 37: 175-82.


14. Kock S, Hopf HB. Incidence and predisposing factors of persistent backache after lumbar catheter epidural anesthesia in a non-obstetrical settingø. Anasthesiol Intensivmed Notfallmed Schmerzther 1998; 33: 648-52.



15. Vandam LD, Dripps RD. Long-term follow-up of patients who received 10,098 spinal anesthetics. IV. Neurological disease incident to traumatic lumbar puncture during spinal anesthesia. J Am Med Assoc 1960; 172: 1483-7.


16. Dahl JB, Schultz P, Anker-Moller E, Christensen EF, Staunstrup HG, Carlsson P. Spinal anaesthesia in young patients using a 29-gauge needle: technical considerations and an evaluation of postoperative complaints compared with general anaesthesia. Br J Anaesth 1990; 64: 178-82.



17. Tarkkila PJ, Heine H, Tervo RR. Comparison of Sprotte and Quincke needles with respect to post dural puncture headache and backache. Reg Anesth 1992; 17: 283-7.

18. Casati A, D'Ambrosio A, De Negri P, Fanelli G, Tagariello V, Tarantino F. A clinical comparison between needle-through-needle and double-segment techniques for combined spinal and epidural anesthesia. Reg Anesth Pain Med 1998; 23: 390-4.


19. Sharma SK, Gambling DR, Joshi GP, Sidawi JE, Herrera ER. Comparison of 26-gauge Atraucan and 25-gauge Whitacre needles: insertion characteristics and complications. Can J Anaesth 1995; 42: 706-10.


20. Brooks RR, Oudekerk C, Olson RL, Daniel C, Vacchiano C, Maye J. The effect of spinal introducer needle use on postoperative back pain. AANA J 2002; 70: 449-52.

21. Seeberger MD, Lang ML, Drewe J, Schneider M, Hauser E, Hruby J. Comparison of spinal and epidural anesthesia for patients younger than 50 years of age. Anesthe Analg 1994; 78: 667-73.

22. Rabinowitz A, Bourdet B, Minville V, Chassery C, Pianezza A, Colombani A, et al. The paramedian technique: a superior initial approach to continuous spinal anesthesia in the elderly. Anesth Analg 2007; 105: 1855-7.


Appendix
Checklist for Postoperative Evaluation for Postdural puncture back pain
1. Do you have Pain at the site of injection?
□ Yes □ No
If yes,
1) Characteristics of these symptoms
□ Dull □ Aching
□ Burning □ Tingling □ Numbness □ Hypesthesia
□ Others
2) Aggravating factor
3) Relieving factor
4) NRS (numeric rating scale)
Unusual sensations
□ Yes □ No
If yes, where?
If pain or any unusual sensations in the legs or buttocks was mentioned, regard as inappropriate for this study.
2. Did you recuperate completely from your anesthetic?
□ Yes □ No
If no, what are your problems?
□ Back pain □ Headache
□ Fatigue □ Nausea/vomiting
□ Dizziness □ Difficulty urinating or defecating