Whole-blood hypocoagulable profile correlates with a greater risk of death within 28 days in patients with severe sepsis
Article information
Abstract
Background
Hypocoagulability and impaired platelet function have been associated with a high risk of death in sepsis. The aim of this cohort study was to determine whether sepsis-induced hypocoagulability and platelet dysfunction (assessed by ROTEM® and MULTIPLATE®, respectively) are increased in sepsis patients who died within 28 days after diagnosis compared with patients who died between 29 and 90 days after diagnosis.
Methods
Consecutive patients admitted to the intensive care unit of Padova University Hospital from March 2015 to March 2018 for severe sepsis were considered. We collected blood samples from all patients to determine ROTEM® and MULTIPLATE® parameters. Each enrolled patient underwent a 90-day follow-up and the mortality rate was recorded.
Results
Of 120 patients, 36 (30%) died within 28 days post-diagnosis (Group A), 23 (19%) died between days 29 and 90 post-diagnosis (Group B), and 61 (51%) were alive after 90 days (survivors). The clotting time in the ROTEM® test and clot formation time in the EXTEM test were significantly more prolonged in Group A than in B. Both groups showed a significantly higher hypocoagulability than survivors in the EXTEM test. MULTIPLATE® platelet function analysis showed that platelet function was significantly lower in Group A than in Group B.
Conclusions
The present study showed that the combination of thromboelastometry and impedance aggregometry may help identifying sepsis patients at high risk of short-term death. Larger studies are warranted to corroborate our results.
Introduction
Despite continually updated sepsis/septic shock guidelines and bundles [1] recommending early diagnosis and prompt initiation of therapy, sepsis remains a challenge worldwide. The overall mortality rate of septic shock ranges from 25–30%, reaching 40–60% among hospitalized patients [1–5]. Sepsis is a complex systemic defense mechanism to an overwhelming infection. The inflammatory response against an invading pathogen is the result of several hemostatic alterations, notably the dysregulation of pro- and anti-coagulant factors [6–9]. Although hypocoagulability appears to accurately predict a fatal outcome in sepsis, conventional coagulation tests are unable to reliably detect sepsis-induced coagulopathy [10]. In addition, recent reports have shown that platelet function and inflammation are tightly linked [10, 11]. Platelets may act as circulating sentinels, binding to infectious agents and presenting them to the reticuloendothelial system [11]. However, experimental findings on platelet aggregation in response to bacteria have yielded conflicting results [11]. Thus, we aimed to evaluate whole-blood coagulation and platelet function in a cohort of patients with severe sepsis via whole-blood thromboelastometry by ROTEM® (Tem International GmbH, Germany) and impedance aggregometry by MULTIPLATE® (Roche Diagnostics GmbH, Germany).
Materials and Methods
All consecutive patients admitted to the intensive care unit (ICU) of Padova University Hospital between March 2015 and March 2018 with a diagnosis of severe sepsis — according to the Surviving Sepsis Campaign criteria [12] — were considered for enrollment. The diagnosis of severe sepsis was established within 6 hours of ICU admission and each septic patient admitted to our ICU was managed according to Sepsis 2 guidelines (before 2016) and Sepsis 3 guidelines (after 2016) [12]. Exclusion criteria were: Younger than 18 or older than 90 years of age, ongoing pregnancy, Child’s C liver disease, New York Heart Association (NYHA) class IV heart disease, chronic kidney disease, metastatic cancer, pre-existing hematological disorders, readmission to ICU, septic shock, ongoing antiplatelet or anticoagulant therapy, and reception of platelets, fresh frozen plasma or other coagulant substances during the 24 hours preceding the enrollment.
All patients were enrolled within six hours after ICU admission. At time of enrollment, i) informed consent was obtained from each patient or their relatives; ii) demographic and clinical data regarding source of infection, comorbidities (e.g., cancer, diabetes) were collected; iii) Sequential Organ Failure Assessment (SOFA) and Japanese Association for Acute Medicine (JAMA) scores were calculated [13,14]; and iv) two BD vacutainers (Becton Dickinson, USA) with sodium citrate 109 mmol/L (3.8% sodium citrate) and one vacutainer with ethylene-diamine-tetra acetic acid 5.4 mg were collected.
All patients (when available) or their relatives received a monthly follow-up telephone call to ascertain the vital status of patients for up to 90 days after the diagnosis of severe sepsis. Thereafter each patient was placed in one of the following groups: Patients in Group A deceased within 28 days post-diagnosis and patients in Group B deceased between days 29 and 90 post-diagnosis. The patients still living 90 days post-diagnosis were labeled ‘Survivors.’
The protocol was approved by the Institutional Ethical Committee on March 19, 2015 (Ref: 3419/AO/15). The study was performed in compliance with the Declaration of Helsinki and in accordance with the STROBE statement (Supplementary Table 1).
The cohort of patients reported in the present study has been partially described in previous studies [15,16].
Laboratory tests
Blood cell counts, coagulation and chemistry parameters were measured using standardized laboratory methods.
Thromboelastometry and platelet function tests were performed within three hours of blood collection on citrated whole blood by trained personnel using an automated ROTEM® delta device (Tem International GmbH, Germany) and a MULTIPLATE® function analyzer (Roche Diagnostics GmbH, Germany) [17] according to standardized procedures and the manufacturer’s recommendations. EXTEM, INTEM, and FIBTEM assays were performed for each enrolled patient and the following parameters were measured: i) clotting time (CT, s), the time from the beginning of the coagulation analysis until an increase in amplitude of 2 mm. CT reflects the activation phase of whole-blood clot formation; ii) clot formation time (CFT, s), the time elapsed for an increase in amplitude of the thromboelastogram from 2 to 20 mm. CFT reflects the propagation phase of whole-blood clot formation; iii) maximum clot firmness (MCF, mm), the maximum amplitude reached in the thromboelastogram; iv) thrombodynamic index (TDI), MCF/CT + CFT to globally assess a patient’s whole-blood coagulation capabilities [16].
The MULTIPLATE® platelet function analysis takes place in a single-use test cell, which incorporates dual copper sensor wires [17]. When activated, platelets adhere to the sensor wires thus increasing the electrical resistance (i.e., impedance). This increase is proportional to the capability of platelets to aggregate on each wire. The results are expressed as Area Under the Curve (AUC, AU*min). The greater the area the more platelets aggregate. Platelets were stimulated in three different ways: i) using adenosine diphosphate (ADP) to activate the ADP receptor (ADP test, Roche Diagnostics GmbH, Germany); ii) via arachidonic acid, checking cyclooxygenase-dependent aggregation (ASPI test, Roche Diagnostics GmbH, Germany); iii) using thrombin receptor-activating peptide-6 (TRAP-6) to activate the thrombin receptor (TRAP test, Roche Diagnostics GmbH, Germany).
Statistical analysis
The sample size calculation was based on previous observations and the following assumptions: i) expected difference in TDI EXTEM between survivors and non-survivors of 0.06 ii) expected standard deviation (SD) of 0.01; iii) power = 90%; and iv) alpha = 0.05. Based on these assumptions, we needed two groups (e.g., survivors and non-survivors) of at least 55 patients each. Categorical variables were described as frequencies, and comparisons were performed with Fisher’s exact test. The normality assumption was assessed with the Shapiro-Wilk normality test. The ANOVA test and the Bonferroni post-hoc analysis were performed for parametric variables. The Kruskal-Wallis test and Dunn's multiple comparisons post-hoc analysis were used for non-parametric variables. In addition, receiver operating characteristic (ROC) analysis was performed for the most meaningful parameters. All statistical analyses were performed with GraphPad Prism 7 (GraphPad Software Inc., USA) and the PAWS Statistics 17.0.2 (SPSS Inc., USA) for Windows.
Results
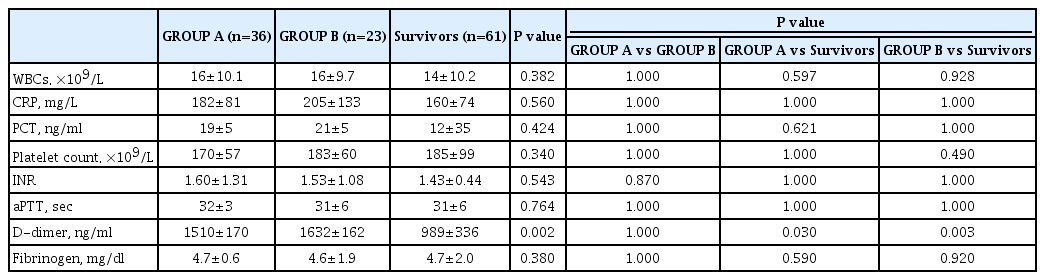
We initially considered a total of 155 subjects. Thirty-five patients were excluded for the following reasons: n = 3 for metastatic cancer, n = 7 for hematological disorders, and n = 25 for ongoing antiplatelet or anticoagulant therapy, leaving 120 participants. Thirty-six (30%) patients died within 28 days of severe sepsis diagnosis (Group A) and twenty-three (19%) patients between days 29 and 90 (Group B). Sixty-one (51%) patients were still living at day 90 (Survivors). The demographic and clinical characteristics of the study population are reported in Table 1. There were no significant differences between groups A and B in terms of gender, age, comorbidities (e.g., cancer, diabetes), cause of sepsis, origin of infection, and SOFA and JAAM scores. Survivors were significantly younger than patients in both groups A and B (P < 0.001 in both comparisons). Regarding traditional coagulation parameters, no significant differences were found between Group A and Group B (Table 2). D-dimer levels were significantly higher in groups A and B compared to Survivors (P = 0.030 and 0.003, respectively).
ROTEM® parameters
ROTEM® parameters are described in Table 3. Both CT and CFT in the EXTEM assay were significantly prolonged in Group A compared to Group B (P = 0.041 and 0.020, respectively). The remaining EXTEM parameters (i.e., MCF and TDI) were similar between Group A and Group B. INTEM and FIBTEM assays revealed no significant differences between Group A and Group B. In the comparison between Group A and Group B vs survivors, all parameters considered in the EXTEM assay revealed a significantly hypocoagulable profile in the former two groups (Table 3). No significant differences were found between Group A and Group B vs Survivors in the INTEM and FIBTEM assays.
MULTIPLATE® parameters
The MULTIPLATE® parameters are reported in Table 4. Platelet function in ADP, ASPI, and TRAP tests was significantly lower in Group A than in both Group B (P = 0.020, 0.007, and 0.005, respectively) and Survivors (P = 0.006, 0.015, and 0.004, respectively). No differences were found between Group B and Survivors. Furthermore, an ROC analysis revealed a significantly worse platelet dysfunction in Group A than in both Group B and Survivors (P ≤ 0.006 for both groups in all reagents considered) (Table 5).
Discussion
Sepsis-induced coagulopathy is characterized by predominant activation of the tissue factor pathway with a remarkable consumption of coagulation factors, platelet activation, and fibrinolysis [6–9,18]. However, traditional coagulation tests (i.e., prothrombin time, activated partial thromboplastin time, and platelet count) have shown several limitations in their ability to reliably and consistently detect coagulation disorders in sepsis [1,3,18–20]. Our findings confirmed that only a high age and D-dimer among standard laboratory tests appeared to BE differentbetween non-survivors and controls.
Therefore, we investigated the use of thromboelastometry (ROTEM®) in sepsis, a promising point-of-care test that was shown to be effective as a rapid global assessment of hemostasis in whole-blood samples, allowing the assessment of each stage of the coagulation process in bleeding patients [21–24]. We also examined the potential correlation between a higher risk of short-term death and severe hypocoagulability. Finally, we assessed sepsis-induced platelet dysfunction by using impedance aggregometry MULTIPLATE®), a promising technique still under investigation in sepsis [11]. We were able to confirm that both non-survivors at 28 days (Group A) and non-survivors between days 29 and 90 (Group B) exhibited more hypocoagulable profiles compared to Survivors (i.e., longer CT, longer CFT, and reduced MCF and TDI in the EXTEM assay). These parameters have been widely studied in the literature, and each parameter measures a specific phase of the coagulation cascade which is differently influenced by coagulation factors, fibrinogen, platelets, or the fibrinolytic system [18,22–24]. However, the prognostic value of these parameters remains unclear. In fact, we previously showed that only the novel TDI — the ratio between MCF, CT, and CFT — is an independent predictor of long-term mortality owing to its ability to assess the patient’s global coagulation capabilities [16]. Based on this finding, we hypothesized that increased hypocoagulability may correlate with a higher risk of short-term death and that traditional coagulation tests carry no prognostic value in non-survivors at 28 days vs 90 days. We found that non-survivors at 28 days had longer CT and CFT values in the EXTEM assay vs. non-survivors between 29 and 90 days, linking higher hypocoagulability to the risk of death within 28 days.
Some reports have indicated that alterations in hemostasis and blood coagulation may occur as early as 60 minutes after induction of endotoxemia. Sepsis-induced coagulopathies have been linked to higher endotoxin activity, increased release of biomarkers of endothelial injury, meaningful changes in the coagulation process, and higher mortality risk [25–27]. Specifically, CFT has been previously described as the most sensitive tool for the rapid detection of hypocoagulability though no differences between short and long-term mortality have been demonstrated to date [25]. Moreover, CFT relies on clotting factors and platelet function [18] and it is well established that thrombocytopenia is an important predictor of ICU mortality [28]. However, we found no differences in platelet count between cases and controls, which informed our decision to use the more accurate MULTIPLATE® device to conduct an in-depth analysis of sepsis-induced platelet dysfunction. We found that, despite similar platelet counts across groups, aggregation in the ADP, ASPI, and TRAP tests was significantly impaired in Group A compared to Group B and Survivors. Platelet function was similar in the latter two groups.
Some of our MULTIPLATE® findings have been partially described in previous studies but no data are available pertaining to differences between long and short-term mortality [11,29,30]. Adamzik et al. [11] compared sepsis patients to post-operative patients and concluded that impedance aggregometry was a better predictor of 30-day survival than conventional biomarkers such as platelet count, although they did not assess long-term mortality. Similar results were obtained by Davies et al. [29] who analyzed 106 adults and reported a more significantly impaired platelet aggregation in patients with severe sepsis/septic shock (compared with SIRS/sepsis without complications) and in non-survivors at 28 days. The authors concluded that reduced platelet aggregometry responses were significantly associated with morbidity and mortality in sepsis and SIRS patients. MULTIPLATE® was shown to be a valid point-of-care test in sepsis patients with overt disseminated intravascular coagulation, a life-threatening complication that often occurs in critically ill patients [30]. However, no investigation discriminated between short-term and long-term non-survivors. In clinical practice, the risk stratification of mortality in adults with sepsis could be vital.
In addition, we opted to enroll all patients upon admission to ensure that early differences in coagulative profiles and platelet function among severe-sepsis subjects would be accounted for, rather than studying changes over time, which could be an interesting starting point for future investigations [27]. We would like to acknowledge some of the limitations of our study: i) the sample size, albeit the study population was highly homogeneous, was restricted by extensive exclusion criteria; ii) ROTEM® and MULTIPLATE® are not readily available in most health facilities; iii) this was an observational study, thus making it impossible to independently assess the causal relationship between ROTEM®/MULTIPLATE® results and outcomes; iv) the thromboelastometry method and impedance aggregometry are non-standardized tests that yield highly variable results across patients. Therefore, the present study should be considered as a hypothesis-generating effort rather than an attempt to provide definitive answers as to the clinical utility of ROTEM® and MULTIPLATE® in every-day clinical practice. Further large-scale prospective investigations are warranted to support our findings.
The present study showed that the combination of thromboelastometry and impedance aggregometry may help identifying sepsis patients at high risk of short-term death. Non-survivors at 28 days more frequently exhibited a higher level of hypocoagulability compared to non-survivors at 90 days. In particular, CT and CFT in the EXTEM assay resulted significantly prolonged and platelet aggregation was meaningfully impaired in all reagents considered in patients who died within 28 days.
Notes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Annalisa Boscolo (Conceptualization; Data curation; Writing – original draft; Writing – review & editing)
Luca Spiezia (Conceptualization; Formal analysis; Investigation; Methodology)
Elena Campello (Conceptualization; Formal analysis; Methodology)
Diana Bertini (Conceptualization; Data curation)
Vittorio Lucchetta (Conceptualization; Data curation)
Eleonora Piasentini (Data curation)
Alessandro De Cassai (Conceptualization; Formal analysis)
Paolo Simioni (Conceptualization; Supervision; Validation; Writing – original draft; Writing – review & editing)
Supplementary Materials
Strengthening The Reporting of OBservational Studies in Epidemiology (STROBE) Checklist