Ultrasound-guided percutaneous intercostal cryoanalgesia for multiple weeks of analgesia following mastectomy: a case series
Article information
Abstract
Background
Acute post-mastectomy pain is frequently challenging to adequately treat with local anesthetic-based regional anesthesia techniques due to its relatively long duration measured in multiple weeks.
Case
We report three cases in which preoperative ultrasound-guided percutaneous intercostal nerve cryoneurolysis was performed to treat pain following mastectomy. Across all postoperative days and all three patients, the mean pain score on the numeric rating scale was 0 for each day. Similarly, no patient required any supplemental opioid analgesics during the entire postoperative period; and, no patient reported insomnia or awakenings due to pain at any time point. This was a significant improvement over historic cohorts.
Conclusions
Ultrasound-guided percutaneous cryoanalgesia is a potential novel analgesic modality for acute pain management which has a duration that better-matches mastectomy than other currently-described techniques. Appropriately powered randomized, controlled clinical trials are required to demonstrate and quantify both potential benefits and risks.
Poorly controlled post-mastectomy pain remains a challenge often leading to persistent postsurgical pain lasting months to years [1]. Due to its typical duration of multiple weeks, post-mastectomy pain is frequently challenging to adequately treat with local anesthetic-based regional anesthesia techniques which provide multiple hours or days of analgesia (e.g., single injection nerve blocks or continuous nerve blocks, respectively). Ultrasound-guided percutaneous cryoneurolysis is an alternative regional analgesic modality that reversibly induces peripheral nerve Wallerian degeneration using extremely cold temperatures, yet spares the endo-, peri-, and epineurium along which the nerve regenerates at approximately 1–2 mm/d. The result is a temporary sensory and motor nerve block with a duration measured in weeks and occasionally months without any delivery device to manage or infusion pump to remove [2]. While described extensively in the chronic pain literature [3], recently published case reports support the use of ultrasound-guided percutaneous cryoneurolysis for the treatment of acute postoperative pain [2,4–6]. Given its analgesic duration roughly corresponds to that of post-mastectomy pain, cryoanalgesia is a possible analgesic option. We now report three cases in which preoperative ultrasound-guided percutaneous intercostal nerve cryoneurolysis was performed to treat pain following mastectomy.
Case Reports
All patients (n = 3) provided written, informed consent for unidentifiable inclusion in subsequent publications. The University of California San Diego Institutional Review Board (San Diego, CA) waives review requirements for case reports. A curvilinear ultrasound probe (C60, Sonosite, USA) was used for all procedures. All patients had an ipsilateral ultrasound-guided paravertebral catheter inserted at either T3 or T4 lateral approximately 2.5 cm from the midline using a technique previously described [7], followed by an injection into the perineurial catheter of ropivacaine 0.5% with epinephrine (1 : 400,000) in divided doses with gentle aspiration every 2–3 ml. Bilateral catheters were inserted for bilateral mastectomy procedures.
Immediately after placement of the paravertebral catheter(s), ultrasound-guided percutaneous cryoanagelsia was performed on four proximal ipsilateral intercostal nerves: T2–5, approximately 5 cm from the midline. The sterile field and ultrasound transducer cover used for the perineural catheter insertion were used for the subsequent cryoneurolysis procedure. The intercostal artery was identified caudad to the respective rib in the parasagittal plane with a short-axis view. Lidocaine 1% was injected subcutaneously to ensure topical anesthesia. A 14-gauge angiocatheter was advanced towards the target nerve leaving the tip within the internal intercostal muscle prior to reaching the nerve itself. The angiocatheter needle was withdrawn leaving the angiocatheter in place.
The 90 mm cryoprobe (Iovera Smart Tip, Myoscience, USA) was introduced via the angiocatheter and advanced to the T2 intercostal nerve under in-plane ultrasound guidance (Fig. 1). Three cycles of 2 min of freezing followed by a 1-min thawing period were applied and the probe subsequently withdrawn. This procedure was repeated on the next three intercostal nerves. For bilateral mastectomies, the cryoneurolysis procedure was repeated on the contralateral side.

Ultrasound image of cryoprobe advancing towards intercostal nerve. (A) proximal short-axis view (5 cm lateral to spinous process) of ribs where intercostal nerve blocks are performed, (B) rendering of Fig. 1A labeling ribs, pleura, and intercostal artery (red circle), (C) view with cryoprobe in-plane next to target, (D) rendering of Fig. 1C with trocar labeled, (E) view with ice-ball formation at tip of cryoprobe, (F) rendering of Fig. 1E with ice-ball labeled (blue circle).
All patients received a general anesthetic without complications. Within the recovery room the perineural catheter(s) were attached to portable pump(s) which infused ropivacaine 0.2% 8 ml/h basal with an optional patient-controlled 4 ml bolus available every 30 min (every 60 min for bilateral catheters). Catheters were removed the morning of postoperative day 2 prior to home discharge, and patients given a prescription for oxycodone (5 mg tablets) to be taken if needed. All patients were called by telephone on postoperative days 1–4, 7, 14, 21, and 28. Patient #1 was a 46-year-old woman with hormone receptor positive, Her2-negative breast cancer who underwent left-sided mastectomy and received a T4 paravertebral catheter. Patient #2 was a 45-year-old woman with hormone receptor positive, Her2-negative invasive ductal carcinoma who underwent left-sided mastectomy with axillary sentinel node biopsy who received a T3 paravetebral catheter. Patient #3 was a 47-year-old woman with hormone receptor negative breast cancer who underwent bilateral mastectomy with left-sided axillary node biopsy and received bilateral T3 paravertebral catheters.
Across all postoperative days and all three patients, the mean pain score on the numerical rating scale (NRS) was 0 for each day (Fig. 2 which includes published controls for comparison). Similarly, no patient required any supplemental opioid analgesics in the entire postoperative period (Fig. 3 which includes published controls for comparison). Similarly, no patient reported insomnia or awakenings due to pain at any time point. No complications related to cryoanalgesia were reported in any patient.
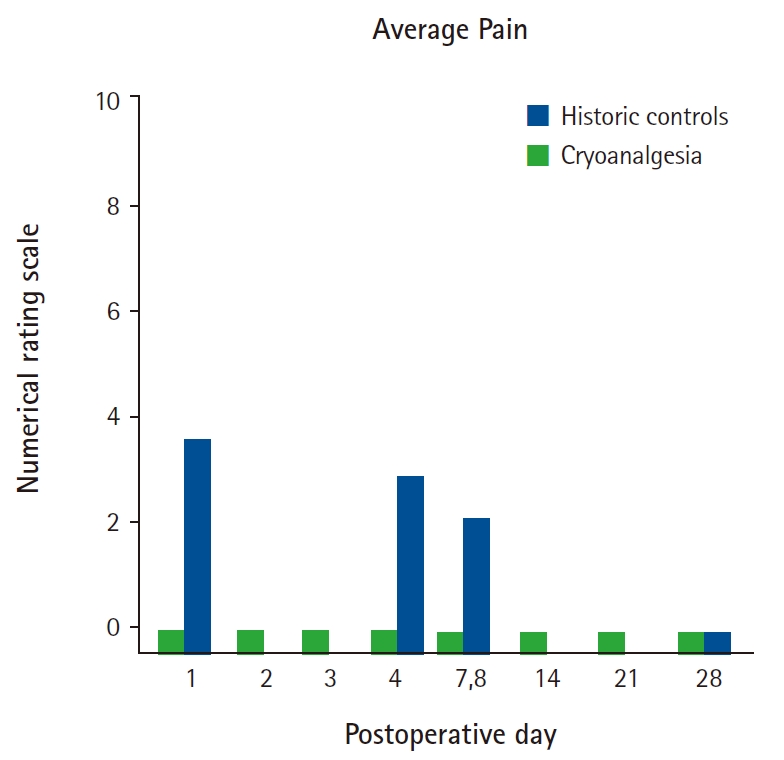
Bar plot demonstrating mean pain scores on the numerical rating scale on various postoperative days in the cryoanalgesia cohort versus historical cohort (patients not receiving cryoanalgesia). Both cohorts had thoracic paravertebral catheters with a ropivacaine infusion for 3 days.
Discussion
We describe three cases in which preoperative ultrasound-guided percutaneous intercostal cryoanalgesia provided a nearly pain-free postoperative course following uni- and bilateral mastectomies without requiring supplemental oral analgesics. This is noteworthy considering this surgical procedure usually results in pain that outlasts a single-injection nerve block and breaks through even a continuous perineural local anesthetic infusion, resulting in supplemental opioid requirements [7].
We provided a continuous paravertebral nerve block to the three patients described in this report since there were no similar previously-published cases (that we are aware of) to suggest the degree of cryo-induced analgesia that might be expected, and we wanted to provide our current standard-of-care analgesics. In addition, our primary aim was to provide potent analgesia following perineural catheter withdrawal prior to discharge on postoperative day 2, and not replace the single-injection and/or continuous nerve blocks. However, as it happens, the cryoneurolysis appears to have provided significant benefit even during the ropivacaine infusion since these three patients reported almost no pain at all, while published controls from our own institution and receiving a similar continuous paravertebral ropivacaine infusion reported a median (interquartile) NRS of 3.6 (2.0–4.0) the day following mastectomy [1]. Apparent related benefits of the cryoanalgesia may be found in the increased quality of sleep, with these three patients reporting no insomnia due to pain, compared with 30%, 27%, 33%, and 20% for historic controls the nights of postoperative days 0, 3, 7, and 28, respectively [7].
Percutaneous ultrasound-guided cryoanalgesia has been previously described for a few acute pain indications, both perioperative and unrelated to surgery [2,4–6]. However, its application to mastectomy appears to be particularly suitable due to various factors, including (1) the near-total coverage of the surgical site with the blocks; (2) the relative insignificance of cryo-induced sensory and motor block; and, (3) the similar durations of post-mastectomy pain and cryoneurolysis. Importantly, the development of persistent postsurgical pain is associated with the level of pain experienced in the immediate postoperative period; and multiple studies have demonstrated decreased chronic pain with improved analgesia in the 2–3 d following mastectomy [8,9]. Considering the duration of cryoanalgesia will usually match or extend beyond post-mastectomy pain—offering the possibility of a relatively pain-free surgical experience—this modality has the potential to significantly decrease the risk of persistent postsurgical pain.
Direct comparisons with continuous peripheral nerve blocks are unavailable, but some theoretical benefits of cryoneurolysis include an ultra-long duration of action, no catheter insertion/removal, no required infusion management/oversight, the lack of an infusion pump and anesthetic reservoir to carry, a considerably lower risk of infection, and no risk of local anesthetic toxicity, catheter dislodgement, or fluid leakage [10]. Risks include bleeding, bruising, and—if the ice ball involves the skin—frostbite, alopecia, depigmentation, and/or hyperpigmentation. Other disadvantages include the requirement of an initial capital investment for the cryoneurolysis machine (although subsequent per-patient costs are relatively low) [11]; a somewhat unpredictable duration of action [12]; and frequent dense sensory and motor blocks (less consequential following mastectomy, but significant for other surgical procedures and treated nerves) [3].
With over 50 years of clinical use, the published literature suggests cryoneurolysis has a level of safety surpassing traditional local anesthetic-based peripheral nerve blocks. However, it is noteworthy that two randomized, controlled investigations reported a statistically significant increase in the incidence of neuropathic pain for cryoneurolysis administered via the surgical incision in subjects at 8 weeks (resolving by 6 months) [13], 6 months, and 1 year following open thoracotomy [14]. In contrast, the majority of randomized, controlled trials of cryoneurolysis via the surgical incision did not report any increased risk of persistent postoperative pain, although a few additional small studies did note a possible association that did not reach statistical significance [15]. The reasons for these discrepancies remain unknown, and due to a myriad of confounding variables among studies, no causal inferences can be made without additional data. Considering the lack of reports of chronic pain following cryoneurolysis for other indications [3], this may be an issue related exclusively to thoracotomy—possibly related to the double-crush theory.
In summary, ultrasound-guided percutaneous cryoanalgesia is a potential novel modality for acute pain management following mastectomy. Benefits may potentially extend beyond the duration of the cryoneurolysis with a decrease in the incidence and severity of chronic postoperative pain. Appropriately powered randomized, controlled clinical trials are required to demonstrate and quantify both potential benefits and risks.
Notes
Funding Statement
Myoscience (Fremont, USA) provided an unrestricted research grant and the cryoneurolysis devices used in these case reports. The investigators retained full control of the investigation, including the treatment protocol, data collection, data analysis, results interpretation, and manuscript preparation. Myoscience was not consulted regarding the contents of this manuscript. Drs. Gabriel, Finneran, Swisher, Said, Sztain, Khatibi, and Ilfeld: The University of California has received funding and product for other research projects from Myoscience and Epimed (Farmers Branch, USA); infusion pump manufacturer Infutronics (Natick, USA); and a manufacturer of a peripheral nerve stimulation device, SPR Therapeutics (Cleveland, USA). Dr. Ilfeld: In addition to the above, Dr. Ilfeld’s institution has received funding from a manufacturer of a long-acting bupivacaine formulation, Heron Pharmaceuticals, USA.
Conflicts of Interest
Drs. Wallace and Hosseini: No potential conflict of interest relevant to this article was reported.
Author Contributions
Rodney Allanigue Gabriel (Conceptualization; Data curation; Formal analysis; Methodology; Writing – original draft; Writing – review & editing)
John J. Finneran (Conceptualization; Writing – original draft; Writing – review & editing)
Matthew W. Swisher (Conceptualization; Writing – original draft; Writing – review & editing)
Engy T. Said (Conceptualization; Formal analysis; Writing – original draft; Writing – review & editing)
Jacklynn F. Sztain (Writing – original draft; Writing – review & editing)
Bahareh Khatibi (Writing – original draft; Writing – review & editing)
Anne M. Wallace (Conceptualization; Methodology; Writing – original draft; Writing – review & editing)
Ava Hosseini (Methodology; Writing – original draft; Writing – review & editing)
Andrea M. Trescot (Conceptualization; Methodology; Writing – original draft; Writing – review & editing)
Brian M. Ilfeld (Conceptualization; Data curation; Methodology; Writing – original draft; Writing – review & editing)

