Postoperative nausea and vomiting in patients undergoing colorectal surgery within an institutional enhanced recovery after surgery protocol: comparison of two prophylactic antiemetic regimens
Article information
Abstract
Background
Enhanced recovery protocols (ERP) provide optimal perioperative care for surgical patients. Postoperative nausea and vomiting (PONV) is common after colorectal surgery (CRS). We aim to compare the efficacy of aprepitant to a cost-effective alternative, perphenazine, as components of triple antiemetic prophylaxis in ERP patients.
Methods
Patients who underwent ERP CRS at a single institution from July 2015 to July 2017 were evaluated retrospectively. Only subjects who received aprepitant (Group 1) or perphenazine (Group 2) preoperatively for PONV prophylaxis were included. Patient characteristics, simplified Apfel PONV scores, perioperative medications, and PONV incidence were compared between the groups. PONV was defined as the need for rescue antiemetics on postoperative days (POD) 0–5.
Results
Five hundred ninety-seven patients underwent CRS of which 498 met the inclusion criteria. Two hundred thirty-one (46.4%) received aprepitant and 267 (53.6%) received perphenazine. The incidence of early PONV (POD 0–1) was comparable between the two groups: 44.2% in Group 1 and 44.6% in Group 2 (P = 0.926). Late PONV (POD 2–5) occurred less often in Group 1 than Group 2, respectively (35.9% vs. 45.7%, P = 0.027). After matching the groups for preoperative, procedural, and anesthesia characteristics (164 pairs), no difference in early or late PONV could be demonstrated between the groups.
Conclusions
The incidence of PONV remains high despite most patients receiving three prophylactic antiemetic medications. Perphenazine can be considered a cost-effective alternative to oral aprepitant for prophylaxis of PONV in patients undergoing CRS within an ERP.
Introduction
Enhanced recovery protocols (ERP) are designed to provide optimal perioperative care for patients undergoing surgery. Postoperative nausea and vomiting (PONV) is a common problem among patients undergoing surgery as the incidence varies from 30% to as high as 80% in various surgical procedures [1–3]. Furthermore, PONV is a contributor to increased post-anesthesia care unit (PACU) length of stay, increased hospital costs, and decreased patient satisfaction [4,5].
Risk factors for PONV include sex, history of PONV or motion sickness, smoking status, age, anesthesia type, duration of anesthesia, use of volatile anesthetics including nitrous oxide, type of surgery, and opioid use. Because patient physiology and surgical type cannot be changed, current guidelines and strategies focus on the alteration of the anesthetic plan to help decrease the incidence of PONV, including the reduction or exclusion of opioids and volatile anesthetic agents [1,3]. Additionally, the use of prophylactic antiemetics administered preoperatively or intraoperatively is a common strategy to reduce PONV. Among the most commonly used antiemetics are 5-HT3 antagonists such as ondansetron, steroids such as dexamethasone, neurokinin antagonists such as aprepitant, phenothiazine antipsychotics such as perphenazine, and anticholinergic pharmacotherapy such as scopolamine. Although it is known that the use of antiemetics is effective at preventing PONV, controversy exists regarding the best antiemetics for prevention.
At our institution, the ERP for patients undergoing colorectal surgery (CRS) included several factors including but not limited to preoperative hydration, multimodal analgesia with limitation of opioids, and the use of preoperative and intraoperative antiemetics. Aprepitant was the favored preoperative oral antiemetic utilized for patients undergoing CRS under the ERP at our center but was substituted by perphenazine because of substantially higher costs associated with aprepitant. Given the drastic difference in costs, we aimed to evaluate if there was a difference between the two medications in their antiemetic efficacy. We hypothesized that perphenazine is non-inferior to aprepitant as a preoperative prophylactic antiemetic for patients undergoing CRS within an ERP.
Materials and Methods
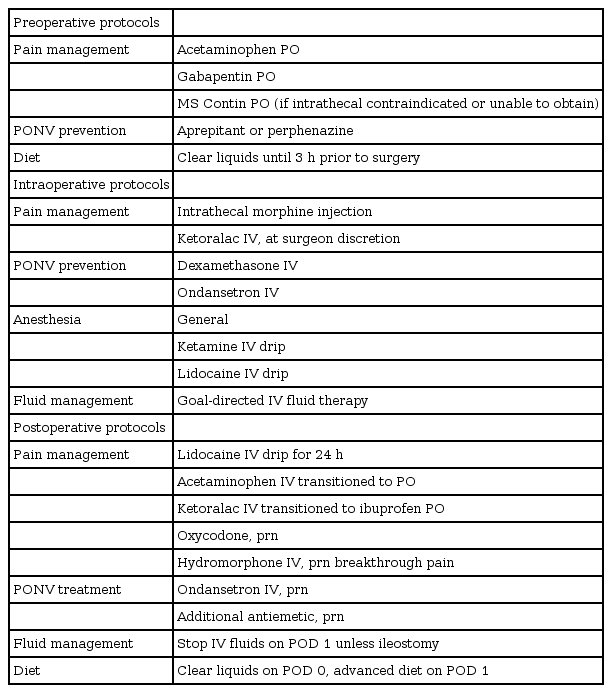
All patients who underwent CRS via an ERP from July 2015 to July 2017 were included. The institutional ERP is described in Table 1. Patient demographics, medication use, and other clinical variables were abstracted from the electronic medical record. Simplified Apfel scores, a measure of PONV risk, were calculated for each patient. Patients who had CRS within an ERP between July 2015 and February 2016 received oral aprepitant 40 mg as the standard preoperative antiemetic medication 1–2 h before surgery; the ERP was altered in February 2016 to use oral perphenazine 8 mg as the standard preoperative antiemetic medication before surgery. Group 1 was defined as patients who received preoperative aprepitant only, and Group 2 was defined as patients who received preoperative perphenazine only. Patients who received more than one oral preoperative antiemetic were excluded from the analysis to reduce potential confounding, therefore, excluding any combination of the following medications: aprepitant, perphenazine, and scopolamine. All patients also received dexamethasone 4 mg after induction of anesthesia and ondansetron 4 mg before emergence for PONV prophylaxis. This study was approved by the University of Pittsburgh Institutional Review Board.
The primary outcome measure was the incidence of PONV. PONV was evaluated by the need for at least one rescue antiemetic on postoperative days (POD) 0 through 5. Early PONV was defined as the need for at least one rescue antiemetic anytime between POD 0 and 1 and late PONV was defined as the need for at least one rescue antiemetic anytime between POD 2 and 5. Ondansetron 4 mg was used as the primary rescue antiemetic and prochlorperazine 5–10 mg and/or promethazine 12.5–25 mg were utilized in PONV refractory to ondansetron. Each antiemetic was evaluated for their incidence and frequency of use. Postoperative use of opioid medications (fentanyl, oxycodone, hydromorphone, and morphine) was noted for both groups. Each opioid pain medication dose was converted into intravenous morphine equivalents (IV ME) to evaluate total opioid consumption. As prolonged postoperative ileus may alter the incidence of PONV, we noted and compared the incidence between the two groups. Continuous parameters were analyzed using the Mann-Whitney U test, and all dichotomous variables were analyzed by Chi2 test. In order to compare patients with similar patient characteristics, anesthesia technique, and other operative parameters, Group 1 patients were propensity matched to Group 2 patients for an additional analysis. Matching was done using a predictive score derived from logistic regression with a caliper distance of less than 0.05, yielding 164 matched pairs (1 : 1 matching). Baseline characteristics and PONV outcomes were then compared between the matched groups. An alpha level of 0.05 was used to determine statistical significance. SPSS (IBM Corp., USA) version 25.0 was used for statistical analysis.
Results
A total of 597 patients underwent CRS during the study period. Sixty-nine patients received neither aprepitant nor perphenazine, 22 patients received transdermal scopolamine patch, eight patients received both perphenazine and aprepitant; all of these patients were excluded from the study. Therefore, 498 met the inclusion criteria for our study. Group 1 consisted of 231 (46.4%) patients who received aprepitant only while Group 2 consisted of 267 (53.6%) who received perphenazine only as oral preoperative antiemetic prophylaxis before surgery.
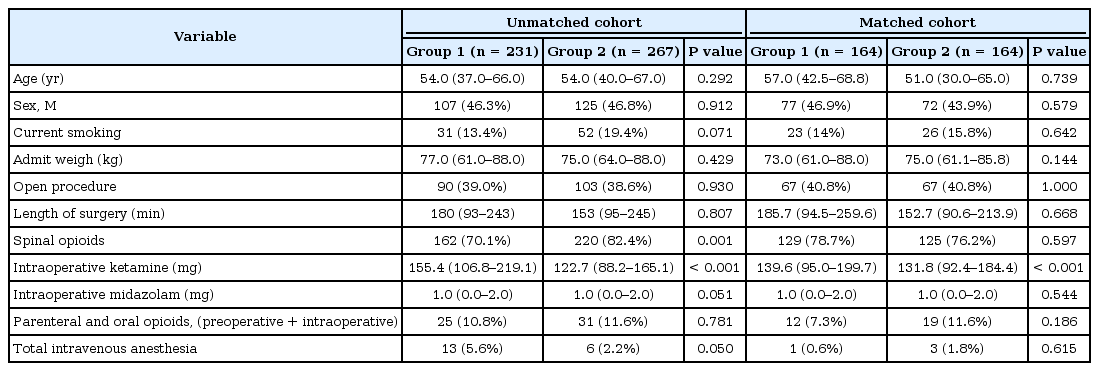
Patients in Group 1 received a significantly higher dose of intraoperative IV ketamine (P < 0.001) and a trend toward increased use of total intravenous anesthesia (TIVA) (P = 0.06) than patients in Group 2. However, TIVA was used in very few patients in both groups: 13 patients in Group 1 (5.6%) and 6 patients (2.2%) in Group 2. All others received inhalational anesthesia for maintenance anesthesia during the surgery. Group 2 patients had a trend toward a higher frequency of smoking history (P = 0.071) and received spinal opioids more often (P = 0.001) than Group 1 patients (Table 2). The distribution of simplified Apfel scores within the two groups was comparable (Table 3).
As baseline patient characteristics and anesthesia techniques (spinal opioids, smoking history, TIVA, and midazolam) can influence the incidence of PONV, propensity matching was performed to obtain patients matched for those variables. We did not attempt to match patients for IV ketamine dose, as a protocol change in ketamine dosage (0.6 mg/kg/h to 0.4 mg/kg/h) occurred at the time of the antiemetic protocol change (aprepitant to perphenazine). After matching, only the intraoperative ketamine dose remained significantly different between the two groups; all other factors were comparable and statistically similar.
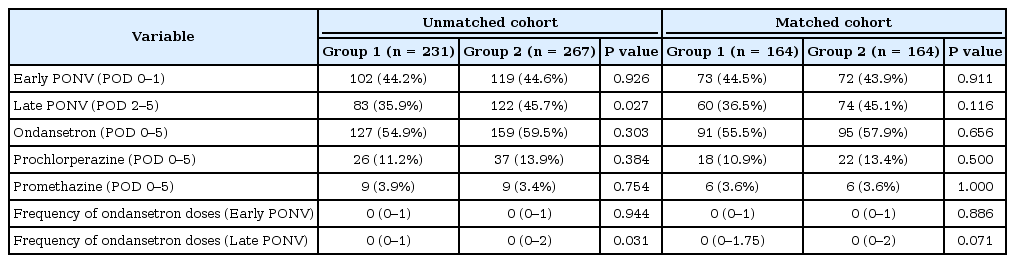
The incidence of early PONV (POD 0–1) was comparable between unmatched and matched patients with 44.2% in Group 1 and 44.6% in Group 2 (P = 0.926), and 44.5% and 43.9% (P = 0.911), respectively. Prophylaxis with aprepitant significantly reduced the incidence of late PONV (POD 2–5) when compared to perphenazine prophylaxis (35.9% in Group 1 vs. 45.7% in Group 2, P = 0.027) in the unmatched cohort. The frequency of ondansetron administration during POD 2–5 was also significantly less with aprepitant prophylaxis in the unmatched cohort (Table 4). For matched pairs, the incidence of both early and late PONV was not significantly different between the two groups. The frequency of each postoperative antiemetic (ondansetron, prochlorperazine, and promethazine) administration for POD 0–5 was comparable between the matched patients (Table 4). Of note, postoperative ileus rates, defined as nasogastric tube insertion or NPO at POD 4, were similar between both unmatched (39.8% vs. 37.8%, P = 0.692) and matched patients (42.7% and 37.8%, P = 0.368).
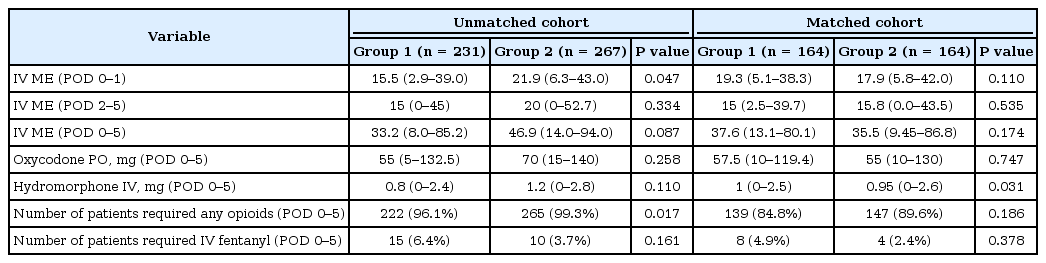
Although there was a significant difference in the utilization of IV hydromorphone between matched patients, overall opioid consumption measured by IV ME and the number of patients requiring opioid analgesics were found to be similar (Table 5).
The cost of aprepitant at our institution is $130 vs. $2 for perphenazine. Therefore, assuming approximately 250 major abdominal CRS ERP surgeries at our single institution in one year, substitution of perphenazine for aprepitant results in a cost savings of $32,000 per year.
Discussion
The incidence of PONV after CRS varies between 12 and 47% in clinical trials [6–8]. These variations could be explained by the type of surgical procedure, anesthesia medications utilized, the methods used to evaluate PONV, the antiemetic prophylaxis protocol, type of postoperative analgesia, and the perioperative feeding methods utilized. Perioperative use of opioids is often considered as a risk factor for PONV and ERP in CRS often utilize multimodal analgesic techniques to minimize opioid analgesics. A meta-analysis of randomized clinical trials utilizing ERP pain management pathways has reported a decrease in the incidence of PONV by 18% [9]. In a multicenter clinical trial of 300 patients undergoing various colorectal procedures, non-opioid analgesia was utilized in 78% of patients and epidural analgesia in 38% of patients [10]. The incidence of PONV was very low (12%) considering the antiemetic prophylaxis was administered only to 27% of patients in their study [10].
We report a higher incidence of PONV (55%–60%) in this study utilizing an ERP for CRS even with triple antiemetic prophylaxis (dexamethasone and ondansetron with either perphenazine or aprepitant). The ERP in this study utilized liberation of fasting, early feeding, and early mobilization, all of which have been associated with decreasing PONV. Minimally invasive surgical techniques whenever feasible were utilized with approximately 60% of patients receiving laparoscopic surgery, which may increase PONV rates as laparoscopy and the creation of pneumoperitoneum is a known risk factor for PONV Intrathecal morphine, intravenous lidocaine and ketamine, acetaminophen, and non-steroidal anti-inflammatory drugs were utilized for analgesia. Postoperative rescue opioid analgesia consisted of oral oxycodone and intravenous hydromorphone in titrated doses with patient-controlled analgesia only reserved for refractory pain. Intraoperative opioids are avoided except in patients with contraindications for intrathecal morphine. The high incidence of PONV could be explained by several factors. First, neuraxial opioids are well known to cause significant PONV [11]. In a meta-analysis of twenty-eight placebo-controlled studies (n = 1,314 patients) utilizing intrathecal morphine, a total of 790 patients received intrathecal morphine and 524 patients received a placebo. There were statistically significant increases in nausea (RR 1.3, 95% CI 1.1 to 1.5; 24 RCTs), vomiting (RR 1.6, 95% CI 1.1 to 2.2; 19 RCTs), and pruritus (RR 2.0, 95% CI 1.6 to 2.4; 25 RCTs) with intrathecal morphine compared with placebo [11]. Second, ketamine’s opioid-sparing analgesic benefits are described in the literature, though the effect of opioid sparing on opioid-related side effects such as PONV is still controversial and a known side effect of ketamine is increased PONV [12–14]. As ketamine is the mainstay of our ERP, this may have contributed to the high incidence of PONV. Third, we utilized mainly inhalational anesthesia except in very high-risk patients for PONV. Total intravenous anesthesia (TIVA) was used only in 19 (3.8%) patients in this study and the use of TIVA with propofol could have reduced the incidence of PONV [15]. Finally, although intraoperative opioid use could be avoided in almost 90% of patients, the use of postoperative opioids was still frequent with more than 95% of patients receiving some rescue opioids during the study period. The use of continuous catheter analgesia with local anesthetics (epidural or peripheral nerve blocks) could have further reduced the need for opioids and thus potentially the PONV rate.
The incidence of late PONV (POD 2–5 days) was also higher (35%–45%) and the concern of delayed PONV was addressed recently [16,17]. Although there are abundant data on PONV occurrence until 24 h postoperatively, delayed PONV and post-discharge nausea and vomiting after ambulatory surgery deserve further investigation [18–20]. One recent large multicenter pragmatic study (DREAMS trial) involved 1350 participants and evaluated PONV and rescue medication use for 120 h postoperatively in major bowel surgery [21]. The incidences of antiemetic use at 24, 72, and 120 h were 39%, 52%, and 41% in the dexamethasone group and 52%, 63%, and 42% in the standard of care group without dexamethasone. The incidence was quite similar to what we found in our study. Late PONV can be more relevant in colorectal procedures where there is persistence of gastrointestinal irritation and insults even after the anesthetic effects wear off. For example, the persistence of paralytic ileus (defined as NPO on POD 4 or new postoperative insertion of a nasogastric tube) was present in at least one-third of the patients in both of our groups, and likely contributed to the higher rates of late PONV seen throughout our study. Additional measures to decrease postoperative ileus like utilization of continuous epidural analgesia, peripheral nerve blocks, alvimopan, and optimization of goal-directed fluid therapy regimens might improve the late PONV in this patient population.
Ondansetron—a 5-HT3 antagonist—and dexamethasone are recommended as combination antiemetic prophylaxis in patients at high risk for PONV such as laparoscopic CRS [22]. Since patients undergoing CRS are at higher risk for PONV, we decided to add a third drug for antiemetic prophylaxis. Aprepitant, a neurokinin (NK-3) antagonist, has shown better efficacy in producing complete response against PONV when compared with ondansetron in patients at high risk for PONV and is considered the drug of choice for PONV refractory to other drugs [23–26]. Aprepitant also has the advantage of longer half-life compared to 5-HT3 antagonists [27]. However, as aprepitant is more expensive compared to other antiemetics, we replaced aprepitant with perphenazine, a phenothiazine used for treating depression but also nausea and vomiting [28]. A systematic review of 11 clinical trials (n = 2,081) has shown that perphenazine significantly reduced the incidence of PONV (RR 0.50; 95% CI: 0.37–0.67) compared to placebo and was equally efficacious compared to other antiemetic drugs [29]. Adverse events such as extrapyramidal symptoms were rare with the use of perphenazine [30]. While aprepitant is a relatively new drug with its efficacy proven from well-conducted clinical trials, perphenazine is an older, underappreciated drug with its studies predominantly conducted before 2000 and no studies have compared the two.
To date, there is no study that compares the antiemetic efficacy of perphenazine and aprepitant in an ERP patient population. There was no difference in the need for rescue antiemetics in the early postoperative period (POD 0–1) but there was a trend towards less PONV in the late postoperative period with aprepitant although the difference did not reach statistical significance in the matched cohort. This marginal and questionable late benefit could be related to aprepitant’s longer half-life, which needs to be studied in future prospective studies. This could also be accounted for in patient factors such as infection, which was not measured in our study. Nevertheless, the incidence of PONV is very high necessitating additional strategies for prevention of PONV apart from pharmacological prophylaxis in this population as described above.
Several limitations should be considered while interpreting the results of the study. First, this is a single center retrospective study and the antiemetic efficacy was mainly evaluated by the requirement of rescue antiemetics. The incidence of nausea could not be separated from the incidence of vomiting from the documentation. Second, the predicted risk of PONV could not be reliably estimated retrospectively as the calculated Apfel scores from existing records may underestimate the actual risk without additional documentation of patient reported factors. Third, compliance with ERP components was not measured in this study, which could have affected the incidence of PONV. Fourth, oral antiemetics are typically recommended to be given 1–2 h before surgery. Though our practice is for administration in this interval of time, the timing of administration for perphenazine and aprepitant was not monitored and could have affected antiemetic efficacy and results. Finally, it is not possible to conclude on the antiemetic efficacy of orally administered drugs before surgery unless we include a group without both of these drugs (a placebo group). Nonetheless, we can address the inferiority of two medications.
Despite the above limitations of this retrospective study, we present a comparative analysis of two prophylactic antiemetic medications as components of a triple antiemetic prophylaxis regimen in a large ERP population of CRS patients. The incidence of PONV remained high despite triple prophylaxis and ERP measures (opioid restricted perioperative pain management), possibly related to surgical factors like postoperative ileus. We demonstrate that perphenazine has a similar efficacy as aprepitant for prophylaxis of PONV in this population and can be considered as a cost-effective alternative. Other strategies of PONV prevention such as total intravenous anesthesia and local anesthesia based regional analgesic infusions should be considered for these patients in addition to pharmacological prophylaxis given the overall high rate of PONV.
Notes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Jennifer Holder-Murray (Conceptualization; Investigation; Methodology; Supervision; Validation; Writing – original draft; Writing – review & editing)
Stephen A Esper (Conceptualization; Investigation; Supervision; Validation; Writing – original draft; Writing – review & editing)
Michael L Boisen (Investigation; Validation; Writing – original draft; Writing – review & editing)
Julie Gealey (Data curation; Formal analysis; Project administration; Validation; Writing – original draft; Writing – review & editing)
Katie Meister (Conceptualization; Data curation; Investigation; Project administration; Writing – review & editing)
David S Medich (Investigation; Project administration; Supervision; Validation; Writing – original draft; Writing – review & editing)
Kathirvel Subramaniam (Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing)