 |
 |
|
|
Abstract
Background
Bilateral superficial cervical plexus block (BSCPB) provides good postoperative analgesia, but its effect on anesthetic consumption is unknown. This study evaluated the effects of BSCPB on sevoflurane consumption during thyroid surgery.
Methods
Fifty patients were randomly allocated into groups A and B of 25 each in this prospective double-blind study. Group A received BSCPB with 20 ml 0.25% bupivacaine, whereas group B received 20 ml saline immediately before entropy-guided general anesthesia. Intraoperative hemodynamic parameters, end-tidal sevoflurane concentration, minimum alveolar concentration, and sevoflurane consumption were recorded. Postoperative pain was assessed using a visual analog scale, and the time of the first request for analgesia was noted. All side effects were recorded.
Results
Demographics were comparable. Mean sevoflurane consumption [for 30 min: group A = 7.2 (1.1) ml, group B = 8.8 (2.0) ml, P = 0.001; for 60 min: group A = 13.5 (1.7) ml, group B = 16.5 (3.9) ml, P = 0.002] and mean end-tidal sevoflurane concentration [for 30 min: group A = 1.2% (0.2%), group B = 1.4% (0.2%), P = 0.008; for 60 min: group A = 1.2% (0.1%), group B = 1.4% (0.2%), P = 0.010] were significantly lower in group A. Patients in group A had a longer duration of analgesia [361.6 (79.5) min vs. 151.0 (60.2) min, P < 0.001] compared to those in group B.
General anesthesia is the technique of choice for thyroid surgery. In addition to pain, discomfort while swallowing, a burning sensation in the throat, nausea, and vomiting can be caused by surgery or by general anesthesia [1]. Various attempts have been made to prevent or treat these problems with various modalities, such as opioids and nonsteroidal anti-inflammatory drugs, or with additional locoregional anesthesia techniques [2–5]. General anesthesia is routinely maintained using volatile anesthetic agents [1]. Bilateral superficial cervical plexus block (BSCPB) is a popular regional anesthesia technique because of its feasibility and efficacy. Superficial cervical plexus block alone or in combination with deep cervical plexus block has been advocated for postoperative analgesia or as a sole anesthetic technique in some cases [2,6,7]. Superficial cervical plexus block provides good intraoperative and postoperative analgesia and reduces the amount of postoperative analgesic required [8–10]. When the block is performed before surgery, the ensuing analgesic effect may reduce the requirement for anesthetics. The use of monitors, such as entropy and the bispectral index, may help precisely titrate the anesthetic agents. However, very few studies have assessed the effects of BSCPB on anesthetic consumption, particularly when monitors are used to measure the depth of anesthesia. We investigate whether administering BSCPB reduces sevoflurane consumption and prolongs the duration of postoperative analgesia.
This prospective randomized double-blind study was conducted from June 2015 to November 2015. Approval from the institutional ethics committee (BMCRI/PS/IEC/30/2015-16 dated 5/30/2015) was obtained. Fifty patients (age, 20–60 years) of the American Society of Anesthesiologists grades 1 and 2 who were undergoing elective thyroid surgery under general anesthesia were randomly allocated into two groups of 25 each using a computer-generated randomization sequence (www.random.org). Group A received BSCPB with 20 ml 0.25% bupivacaine, and group B received BSCPB with 20 ml saline. Patients diagnosed with vocal cord palsy by preoperative indirect laryngoscopy and those with a coagulation disorder, a neurological or psychiatric disturbance, hypertension or another cardiac disorder, a thyroid mass extending retrosternally, any gross compression or deviation of the trachea, or any known drug allergies were excluded from the study. Patients scheduled for extensive surgery, such as radical neck dissection, were also excluded from the study.
The preanesthetic examination was composed of a detailed history and systemic examination. A thorough airway examination was conducted, and written informed consent to participate in the study was obtained. The patients were educated regarding the use of a visual analog scale (VAS). Preoperative investigations, including thyroid function tests and a neck X-ray, were performed per the institutional protocol. Indirect laryngoscopy was done in all patients to confirm vocal cord mobility. All patients fasted for 8 h prior to surgery. Patients were premedicated with 150 mg oral ranitidine hydrochloride and 0.25 mg alprazolam the night before the surgery. A 50 mg dose of ranitidine and 4 mg ondansetron were administered intravenously 1 h before the induction of anesthesia.
A Ringers lactate solution infusion was started when the patient was moved into the operating theater. Electrocardiography, peripheral oxygen saturation (SpO2), noninvasive blood pressure, and entropy monitors were connected and basal levels were recorded.
All patients were premedicated intravenously with 1 mg midazolam, 0.2 mg glycopyrrolate, and 2 μg/kg fentanyl. Using the computer-generated random list, we allocated patients randomly to group A or group B. Group A received BSCPB with 20 ml 0.25% bupivacaine (10 ml on each side) after premedication. Patients in group B received BSCPB with 20 ml saline (10 ml on each side). The allocation based on the randomization sequence was concealed using sequentially numbered opaque sealed envelopes. The envelopes were opened just before administration of the block by the principal investigator, and the group allocation was done based on the randomization sequence. The group allocation was not revealed to any of the attending anesthesiologists until the end of the study. The drug solutions were prepared under sterile conditions by an anesthesiologist who was not involved in administering the block or monitoring the patients, as per instructions from the principal investigator. The anesthesiologist administering the block was familiar with the technique and monitored patients intraoperatively and postoperatively. He was unaware of the drug being used or the group allocation. A 5 cm long 23 G, short beveled needle was inserted at the midpoint of the posterior border of the clavicular head of the sternocleidomastoid muscle. After negative aspiration for blood, 4 ml solution was injected at the needle insertion point just below the platysma. Then the needle was redirected cephalad and caudad from the initial point of insertion in a subcutaneous plane, and 3 ml drug solution was injected in each direction after negative aspiration for blood. Safety was ensured by restricting advancement of the needle no more than 5 mm in depth.
After administration of the block, patients were preoxygenated with 100% oxygen for 3 min and then induced with an intravenous injection of propofol 20 mg in 30 s increments until response entropy fell below 60. Mask ventilation was confirmed, and intubation was facilitated with an intravenous injection of 0.1 mg/kg vecuronium. Proper positioning of the endotracheal tube was confirmed, the agent gas monitoring line was connected, and ventilation continued with a Datex Ohmeda Avance S5TM anesthesia workstation ventilator (GE Healthcare, Finland). Anesthesia was maintained with 50% nitrous oxide and oxygen as well as sevoflurane to maintain entropy values of 40–60. The initial fresh gas flow was set to 4 L/min, and the sevoflurane concentration was set to 2%. The fresh gas flow was reduced to 2 L/min once the set dial concentration equilibrated with the inspired concentration of sevoflurane (Fi Sevo 2%). The depth of neuromuscular blockade was monitored using a train of four, which was connected after the induction of anesthesia but just before the administration of the muscle relaxant, and maintained below a count of 2 with vecuronium. Hemodynamic parameters (heart rate, systolic blood pressure, diastolic blood pressure, and mean arterial pressure), SpO2, end-tidal carbon dioxide concentration, and response and state entropy were monitored continuously and recorded at 5 min intervals until the end of surgery. End-tidal sevoflurane concentration and age-adjusted minimum alveolar concentration (MAC) were recorded at 5 min intervals, and the volume of sevoflurane consumed was recorded at 30 min intervals from the agent gas module of the anesthesia workstation. An increase in heart rate or mean arterial pressure of more than 25% of basal (or systolic blood pressure > 150 mmHg) was considered tachycardia and hypertension, respectively, and the inspired concentration of sevoflurane was increased. Fentanyl (1 μg/kg) boluses were administered intravenously if tachycardia and hypertension persisted or if the difference between response and state entropy exceeded 10 despite administration of adequate amounts of sevoflurane (end-tidal sevoflurane > 2%). Hypotension was defined as more than a 25% fall in mean arterial pressure and was treated with 100 ml crystalloid boluses. The amount of propofol and fentanyl used were recorded in both groups. Sevoflurane and nitrous oxide were discontinued at the end of surgery. The neuromuscular blocking agent was reversed with an intravenous injection of 0.5 mg/kg neostigmine and 10 μg/kg glycopyrrolate, and the patient was extubated after complete recovery as assessed by clinical observation and neuromuscular monitoring. Postoperative pain was assessed using the VAS, with 0 representing no pain and 10 representing the worst pain. Hemodynamic parameters and VAS scores for pain were recorded at 30 min intervals for the first 2 h and then every hour until the patient received rescue analgesia. Patients received 1 g paracetamol intravenously as a rescue analgesic once the VAS score was > 4. The duration of analgesia was recorded from the time of block administration to the first request for analgesia during the postoperative period. Patients were not given any other analgesic as a routine medication during the early postoperative period, and once the patient received the rescue analgesic, the study was deemed complete. Vascular puncture during injection, hoarseness of voice, pain at the injection site, and any other side effects were recorded.
The sample size was calculated based on a pilot study involving 10 patients who were not included in the final analysis. The mean (standard deviation [SD]) consumption of sevoflurane was 15.2 (2.1) ml over 60 min from the start of sevoflurane administration after intubation without administration of BSCPB. We hypothesized that BSCPB would reduce anesthetic consumption. Maintaining 80% power for the study and an alpha error of 0.05, to detect at least a 10% difference (effect size, 1.5 ml) in sevoflurane consumption over 60 min, assuming an SD of 2 ml and equal variance in both groups, a minimum of 22 patients were required in each group. We included 25 patients in each group assuming a dropout rate of 10%.
All data were entered into a Microsoft Excel spreadsheet (Microsoft Inc., USA). Categorical data are presented in tabular format. Quantitative data were analyzed for normality using the Shapiro–Wilk test, and the normally distributed parameters are presented as mean (SD), whereas those with a skewed distribution are presented as medians with interquartile ranges. Categorical variables were analyzed using the chi-square or Fisher’s exact test, as applicable. Normally distributed quantitative data were analyzed using Student’s t test, whereas those with a skewed distribution, including the VAS (from 30 min to 3 h), were analyzed using the Mann–Whitney U test. A P value < 0.05 was considered significant. End-tidal sevoflurane concentration at 60 min and sevoflurane consumption at 30 min were log transformed, assessed for normality, and subsequently analyzed using the independent sample t test. Statistical analyses were carried out using SPSS ver. 20 for Windows (IBM Corp., USA).
A total of 53 patients were enrolled in the study. One patient in group A was excluded because of a tracheal injury (loss of fresh gas flow for a few minutes through the injury, and fresh gas flow had to be increased), and two patients in group B were excluded because of a protocol violation (erroneous recording of sevoflurane consumption due to equipment failure; Fig. 1). The demographic data and durations of surgery were comparable between the groups (P > 0.05) and are summarized in Table 1.
The trends in end-tidal sevoflurane concentration are shown in Fig. 2. Mean end-tidal sevoflurane concentration at the end of 30 min and 60 min after the initiation of sevoflurane administration was calculated for each patient, and the average of these averages was taken to obtain the average end-tidal sevoflurane concentration at the end of 30 and 60 min period. The average end-tidal sevoflurane concentrations over 30 min and over 60 min were significantly lower in group A than in group B. The mean sevoflurane consumption rates at the end of 30 min and at the end of 60 min were significantly lower in group A compared to group B. Further assessment with confidence intervals of average end-tidal sevoflurane concentration and sevoflurane consumption at 30 min and 60 min revealed significant decreases in group A compared to group B. Additional fentanyl supplementation was required in one patient in group A and two patients in group B to maintain response entropy < 60 during surgery, whereas eight patients in group A and six patients in group B required additional propofol to maintain response entropy < 60 before intubation. Mean propofol and fentanyl requirements were comparable between the groups. The duration of analgesia was much longer in group A than in group B (Table 2). Median VAS scores were lower in group A (median VAS scores = 0 at 30 min and 60 min and median VAS score = 1 at 2 h) than group B (median VAS scores = 2, 4, and 4 at 30 min, 60 min, and 2 h, respectively; P < 0.001 at 30 min, 60 min, 2 h, and 3 h; Fig. 3). Patients were pain-free for a longer time in group A than in group B.
Intraoperative heart rate; systolic, diastolic, and mean arterial pressures; oxygen saturation; end-tidal CO2; and age-adjusted MAC values were not different between the groups. The entropy values were comparable between the groups. The response and state entropy of group A were comparable to those of group B (Table 3, Figs. 4 and 5).
Vascular puncture was noted in three patients in group A and two patients in group B, requiring withdrawal and redirecting of the needle before the solution was injected. One patient in group A had a hoarse voice that recovered spontaneously after 12 h.
The results of this prospective randomized study show that BSCPB with 20 ml 0.25% bupivacaine administered before induction of general anesthesia during thyroid surgery is associated with a significant decrease in intraoperative sevoflurane consumption when entropy is added to standard intraoperative management.
Utilizing electroencephalography-based monitors, such as entropy, to judge the depth of general anesthesia and the level of hypnosis during surgery results in reduced sevoflurane consumption [11,12]. Previous studies used an automated closed circuit loop to deliver sevoflurane and state entropy to monitor the depth of anesthesia. We used response entropy, which depicts not only the state of anesthesia but also the state of frontalis activity, representing a reflex response to pain in the event of inadequate analgesia. Sevoflurane was controlled manually, which may explain the higher consumption in our study compared to previous ones. Response entropy was chosen because it represents not only the state of the brain but also the degree of reflex suppression, which is a component of balanced anesthesia. We believe that entropy monitoring helped us maintain adequate anesthesia depth in the present study, as none of our patients complained of recalling any intraoperative event when assessed 24 h after surgery.
The volume of sevoflurane consumed was calculated and displayed by an inbuilt algorithm fed into the anesthesia workstation software.
The exact mechanism by which neural blockade results in a reduced requirement for anesthesia is not clear, but neuraxial anesthesia is associated with sedative properties. Sedation has been reported in unpremedicated patients undergoing surgery under spinal anesthesia, and an association between level of sensory block and degree of sedation was observed [13]. Inhibition of tonic afferent spinal signaling to the brain may explain the decrease in alertness level following central neuraxial blockade. Afferentation theory, as delineated by Lanier et al. [14], states that tonic sensory and muscle-spindle activity maintains a state of wakefulness, which is likely integrated via the spinal cord in the reticular activating system. Accordingly, an acute decrease in tonic afferent input would be expected to decrease the level of consciousness and thereby increase susceptibility to anesthetic agents. This could also be one of the reasons why neural blockade reduces anesthetic consumption [15].
Blockade of pain pathways from the site of surgery using regional techniques, such as interpleural, epidural, and caudal blocks, also reduces anesthetic consumption and prolongs analgesia apart from providing stable intraoperative hemodynamics [16–18]. The BSCPB in the present study likely reduced sevoflurane consumption by inhibiting afferent input to the brain.
The possibility of central nervous system (CNS) depression due to absorbed local anesthetics cannot be ruled out. Systemically absorbed local anesthetics have some depressant effects on the CNS. Inadvertent intravenous administration of a large dose of lidocaine results in suppression of cortical activity, and systemic infusion of lidocaine reduces the sevoflurane requirement [19,20]. However, the amount of local anesthetic used in the present study may have been insufficient to induce CNS depression.
The combination of superficial and deep cervical plexus block is expected to provide better analgesia than superficial cervical plexus block alone. The incidence of complications is higher with a deep cervical plexus block, apart from the technique being technically more difficult. Hence, we resorted to a superficial cervical plexus block alone [21].
A similar study involved 162 patients who underwent elective thyroid surgery: 52 received BSCPB (12 ml on each side) with 0.5% bupivacaine, 54 received 0.5% levobupivacaine, and the remainder received normal saline before the induction of general anesthesia. The depth of anesthesia was monitored using the auditory evoked potential. It was observed that mean end-tidal desflurane concentration decreased significantly in both groups compared to the control group, fewer patients in both intervention groups required analgesics, and it took longer before the first analgesic dose was needed postoperatively [22]. In the present study, entropy was used to measure the depth of anesthesia, and the end-tidal concentration and sevoflurane consumed were assessed. The results of our study are similar to the observations in the aforementioned study.
Post-thyroidectomy pain can be moderate to severe, at least during the first 24 h after surgery, which makes it imperative to plan for proper postoperative analgesia. Pain following thyroidectomy can vary from 40 to 70 cm on a 100 cm VAS [23]. BSCPB as a modality of pain relief has shown conflicting results. Some authors observed that BSCPB had a significant postoperative analgesic effect [2,24,25], whereas others did not observe much difference in either the VAS score or the requirement for total patient-controlled analgesia [8,26]. A meta-analysis by Warschkow et al. [27] involving eight randomized controlled trials showed that superficial cervical plexus block was efficient for reducing pain during the first 24 h after surgery, but the magnitude of pain relief was too small to be clinically significant. However, in the present study, we observed a significant reduction in median VAS score and prolonged duration of analgesia. We observed a steady increase in VAS score, as the patients were not given any analgesics until they demanded them. However, all patients received rescue analgesics once they complained of mild pain (VAS ≥ 4). The pressure of saline over nerves and the residual effects of analgesics administered intraoperatively may explain the 150 min of analgesia noted in the control group [28]. Procedural failure and ineffective regional block can be a problem. Unfortunately, there is no good method to confirm procedural success [21], although ultrasound-based techniques seem to improve the success rate [29].
A few limitations of this study should be mentioned. First, the present study did not record recovery characteristics, such as time to extubation or time to discharge from the postanesthesia care unit; hence, it is not possible to comment on the effects of reduced consumption on recovery. But none of the patients in the present study took more than 30 min for a complete recovery as assessed clinically. Second, 24 h analgesic consumption was not recorded, so it is not possible to comment on the effects of the block on postoperative analgesic requirements. In addition, use of the blind landmark-based technique rather than ultrasound guidance to administer the block in this study is another limitation. Future studies should assess the effects of this block on long-term benefits of pain relief and assess whether the reduced anesthetic consumption reflects the recovery pattern.
In conclusion, administration of BSCPB before the induction of anesthesia was associated with reduced sevoflurane consumption and provided analgesia during the early postoperative period.
Acknowledgments
We acknowledge valuable input from Dr. Rakesh Garg, associate professor, anesthesiology (All India Institute of Medical Sciences, New Delhi, India) on the statistical analysis and interpretation of the statistics.
Fig. 1.
CONSORT flow diagram. Group A: BSCPB with Bupivacaine (test group), Group B: BSCPB with saline (control group).
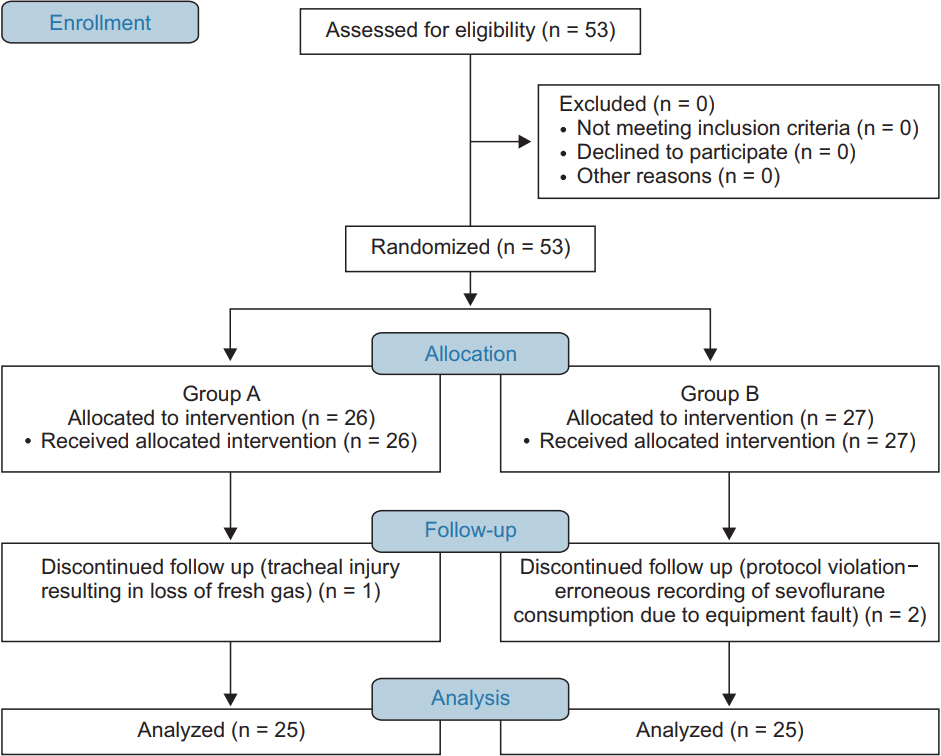
Fig. 2.
Comparison of the trends in end-tidal sevoflurane concentration between the two groups. *The end-tidal sevoflurane concentration was significantly lower in group A compared to group B at 15, 30, 45, and 50 min (P < 0.05). Group A: BSCPB with Bupivacaine (test group), Group B: BSCPB with saline (control group). Post: post-intubation.
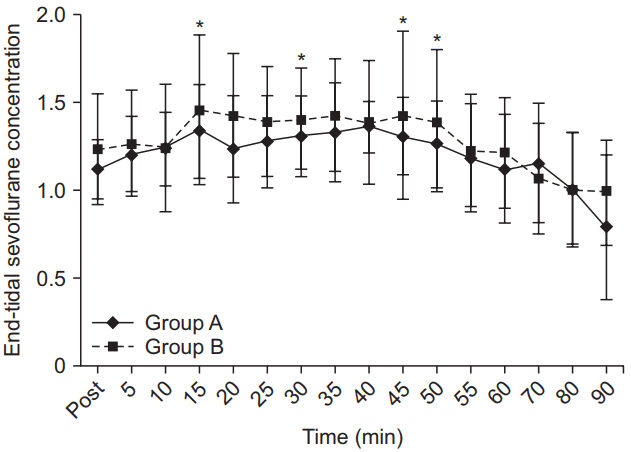
Fig. 3.
Postoperative median visual analog scores. Median visual analog scale (VAS) scores (with interquartile ranges) for both groups. *The scores were significantly lower in group A compared to group B at 30 min, 1, 2, and 3 h (P < 0.001). Analgesia extended to 6 h in group A compared to 3 h in group B.

Fig. 4.
Hemodynamic parameters. Heart rate (HR) and mean arterial pressure (MAP) trends in both groups. Values were comparable at all points of time (P > 0.05). Group A: BSCPB with Bupivacaine (test group), Group B: BSCPB with saline (control group).
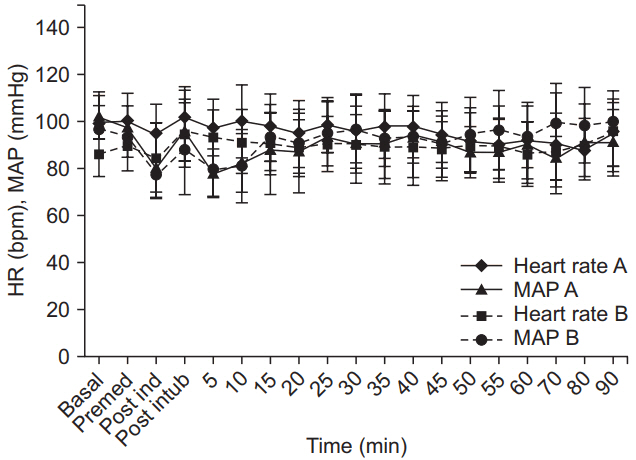
Fig. 5.
Trends in response entropy (RE) in both groups. RE was < 60 at all time points in both groups and comparable between the groups (P > 0.05). Group A: BSCPB with Bupivacaine (test group), Group B: BSCPB with saline (control group).
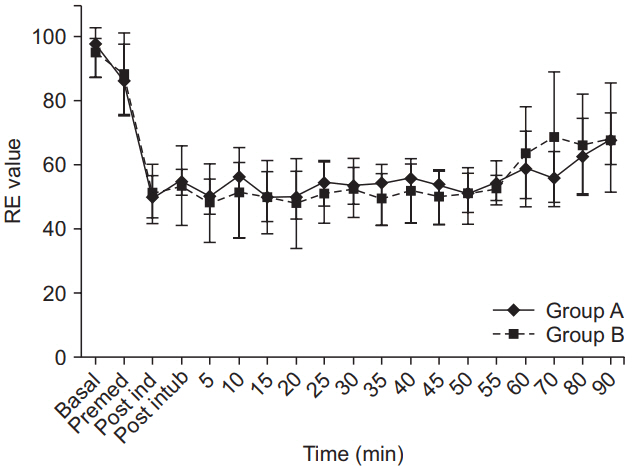
Table 1.
Demographic Characteristics
Table 2.
Comparison of End-tidal Sevoflurane Concentration, Sevoflurane Consumption, Duration of Analgesia, and Propofol and Fentanyl Dose Requirements
| Parameter | Group A (n = 25) | Group B (n = 25) | P value |
|---|---|---|---|
| End-tidal sevoflurane concentration (%) | |||
| 30 min* | 1.2 (0.2) | 1.4 (0.2) | 0.008 |
| 60 min* | 1.2 (0.1) | 1.4 (0.2) | 0.011 |
| 90 min | 1.2 (0.1) | 1.3 (0.2) | 0.084 |
| Mean sevoflurane consumption (ml) | |||
| 30 min | 7.2 (1.1) | 8.8 (2.0) | 0.001 |
| 60 min* | 13.5 (1.7) | 16.5 (3.9) | 0.002 |
| 90 min | 18.7 (2.6) | 20.7 (2.0) | 0.097 |
| Duration of analgesia (min) | 361.6 (79.5) | 151.0 (60.2) | < 0.001 |
| Propofol dose (mg) | 93.6 (23.1) | 95.2 (16.9) | 0.781 |
| Intraoperative fentanyl requirement (μg) | 111.7 (26.4) | 107.4 (21.9) | 0.694 |
Table 3.
Comparison of Mean Peripheral Oxygen Saturation, End-tidal CO2 Concentration, Age-adjusted Minimum Alveolar Concentration, and Response and State Entropy Values
References
1. Bajwa SJ, Sehgal V. Anesthesia and thyroid surgery: the never ending challenges. Indian J Endocrinol Metab 2013; 17: 228-34.



2. Karthikeyan VS, Sistla SC, Badhe AS, Mahalakshmy T, Rajkumar N, Ali SM, et al. Randomized controlled trial on the efficacy of bilateral superficial cervical plexus block in thyroidectomy. Pain Pract 2013; 13: 539-46.


3. Basto ER, Waintrop C, Mourey FD, Landru JP, Eurin BG, Jacob LP. Intravenous ketoprofen in thyroid and parathyroid surgery. Anesth Analg 2001; 92: 1052-7.


4. Karamanlioğlu B, Arar C, Alagöl A, Colak A, Gemlik I, Süt N. Preoperative oral celecoxib versus preoperative oral rofecoxib for pain relief after thyroid surgery. Eur J Anaesthesiol 2003; 20: 490-5.


5. Motamed C, Merle JC, Yakhou L, Combes X, Vodinh J, Kouyoumoudjian C, et al. Postoperative pain scores and analgesic requirements after thyroid surgery: comparison of three intraoperative opioid regimens. Int J Med Sci 2006; 3: 11-3.



6. Mukhopadhyay S, Niyogi M, Dutta M, Ray R, Gayen GC, Mukherjee M, et al. Bilateral superficial cervical plexus block with or without low-dose intravenous ketamine analgesia: effective, simple, safe, and cheap alternative to conventional general anesthesia for selected neck surgeries. Local Reg Anesth 2012; 5: 1-7.



7. Goktas U, Isik Y, Kati I, Aytekin OC, Bartin S. Bilateral superficial and deep cervical plexus block for thyroidectomy in pregnancy. Int J Obstet Anesth 2013; 22: 171.

8. Eti Z, Irmak P, Gulluoglu BM, Manukyan MN, Gogus FY. Does bilateral superficial cervical plexus block decrease analgesic requirement after thyroid surgery? Anesth Analg 2006; 102: 1174-6.


9. Andrieu G, Amrouni H, Robin E, Carnaille B, Wattier JM, Pattou F, et al. Analgesic efficacy of bilateral superficial cervical plexus block administered before thyroid surgery under general anaesthesia. Br J Anaesth 2007; 99: 561-6.



10. Kesisoglou I, Papavramidis TS, Michalopoulos N, Ioannidis K, Trikoupi A, Sapalidis K, et al. Superficial selective cervical plexus block following total thyroidectomy: a randomized trial. Head Neck 2010; 32: 984-8.


11. Wu SC, Wang PC, Liao WT, Shih TH, Chang KA, Lin KC, et al. Use of spectral entropy monitoring in reducing the quantity of sevoflurane as sole inhalational anesthetic and in decreasing the need for antihypertensive drugs in total knee replacement surgery. Acta Anaesthesiol Taiwan 2008; 46: 106-11.


12. El Hor T, Van Der Linden P, De Hert S, Mélot C, Bidgoli J. Impact of entropy monitoring on volatile anesthetic uptake. Anesthesiology 2013; 118: 868-73.


13. Gentili M, Huu PC, Enel D, Hollande J, Bonnet F. Sedation depends on the level of sensory block induced by spinal anaesthesia. Br J Anaesth 1998; 81: 970-1.



14. Lanier WL, Iaizzo PA, Milde JH, Sharbrough FW. The cerebral and systemic effects of movement in response to a noxious stimulus in lightly anesthetized dogs. Possible modulation of cerebral function by muscle afferents. Anesthesiology 1994; 80: 392-401.


15. Eappen S, Kissin I. Effect of subarachnoid bupivacaine block on anesthetic requirements for thiopental in rats. Anesthesiology 1998; 88: 1036-42.


16. Abdulatif M, al-Ghamdi A, Gyamfi YA, el-Sanabary M, al-Metwally R. Can pre-emptive interpleural block reduce perioperative anesthetic and analgesic requirements? Reg Anesth 1995; 20: 296-302.

17. Reinoso-Barbero F, Martínez-García E, Hernández-Gancedo MC, Simon AM. The effect of epidural bupivacaine on maintenance requirements of sevoflurane evaluated by bispectral index in children. Eur J Anaesthesiol 2006; 23: 460-4.


18. Banerjee A, Das B, Mukherjee D, Khanra M. A study of the effect of caudal epidural block on bispectral index targeted propofol requirement in children: a comparative study. J Indian Assoc Pediatr Surg 2015; 20: 77-81.



19. Gaughen CM, Durieux M. The effect of too much intravenous lidocaine on bispectral index. Anesth Analg 2006; 103: 1464-5.


20. Harsoor SS, Rani D, Roopa MN, Lathashree S, Sudheesh K, Nethra SS. Anesthetic sparing effect of intraoperative lignocaine or dexmedetomidine infusion on sevoflurane during general anesthesia. Middle East J Anaesthesiol 2015; 23: 301-7.

21. Pandit JJ, Satya-Krishna R, Gration P. Superficial or deep cervical plexus block for carotid endarterectomy: a systematic review of complications. Br J Anaesth 2007; 99: 159-69.



22. Shih ML, Duh QY, Hsieh CB, Liu YC, Lu CH, Wong CS, et al. Bilateral superficial cervical plexus block combined with general anesthesia administered in thyroid operations. World J Surg 2010; 34: 2338-43.



23. Sonner JM, Hynson JM, Clark O, Katz JA. Nausea and vomiting following thyroid and parathyroid surgery. J Clin Anesth 1997; 9: 398-402.


24. Steffen T, Warschkow R, Brändle M, Tarantino I, Clerici T. Randomized controlled trial of bilateral superficial cervical plexus block versus placebo in thyroid surgery. Br J Surg 2010; 97: 1000-6.


25. Egan RJ, Hopkins JC, Beamish AJ, Shah R, Edwards AG, Morgan JD. Randomized clinical trial of intraoperative superficial cervical plexus block versus incisional local anaesthesia in thyroid and parathyroid surgery. Br J Surg 2013; 100: 1732-8.


26. Herbland A, Cantini O, Reynier P, Valat P, Jougon J, Arimone Y, et al. The bilateral superficial cervical plexus block with 0.75% ropivacaine administered before or after surgery does not prevent postoperative pain after total thyroidectomy. Reg Anesth Pain Med 2006; 31: 34-9.


27. Warschkow R, Tarantino I, Jensen K, Beutner U, Clerici T, Schmied BM, et al. Bilateral superficial cervical plexus block in combination with general anesthesia has a low efficacy in thyroid surgery: a meta-analysis of randomized controlled trials. Thyroid 2012; 22: 44-52.











