Effectiveness of applying continuous positive airway pressure in a patient with paradoxical vocal fold movement after endotracheal extubation: a case report
Article information
Abstract
Paradoxical vocal fold movement (PVFM) is an uncommon upper airway disorder defined as paradoxical adduction of the vocal folds during inspiration. The etiology and treatment of PVFM are unclear. The physician should manage this condition because of the possibility of near complete airway obstruction in severe case of PVFM. We report a case of successful airway management in a patient with PVFM by applying continuous positive airway pressure (CPAP). In this case, PVFM was detected after removing an endotracheal tube from a 67-year-old male who underwent excision of a laryngeal mass. The patient recovered without complications in 1 day with support by CPAP.
Upper airway obstruction after endotracheal extubation is a common complication for an anesthesiologist. Paradoxical vocal fold movement (PVFM) is a rare upper airway disorder that involves paradoxical adduction of the true vocal cords during inspiration. Symptoms of PVFM include dyspnea, inspiratory stridor, and near complete airway obstruction in severe cases [1].
The etiology of PVFM is unclear. Treatments for patients with PVFM are reassurance, benzodiazepines, heliox, speech therapy, psychotherapy, biofeedback, inhaled anticholinergics, an inspiratory valve, continuous positive airway pressure (CPAP), and botulinum toxin [2]. We report a case of successful airway management by applying CPAP in a patient who complained of dyspnea and inspiratory stridor as a result of PVFM that occurred immediately after an endotracheal tube was extubated after vocal cord surgery.
Case Report
A 67-year-old male (height, 165.4 cm; weight, 69.1 kg) with a history of hypertension and hyperthyroidism was scheduled to excise a laryngeal mass because of right vocal fold leukoplakia. The patient has taken oral alprazolam as required for right hand tremor.
The patient's preoperative laboratory test, chest X-ray, pulmonary function test results were unremarkable. Transnasal fiberoptic laryngoscopy taken 2 months prior to the surgery showed right vocal cord palsy (Fig. 1A). He had hoarseness but no signs of dyspnea.
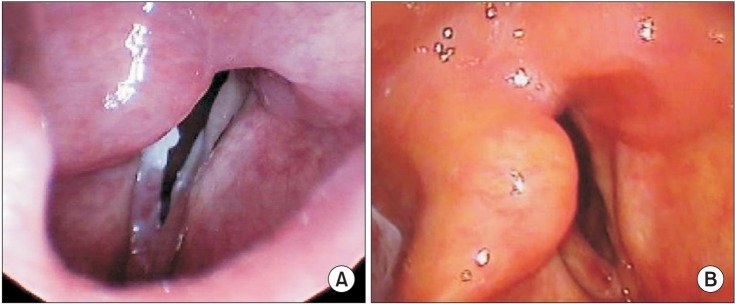
(A) Transnasal fiberoptic laryngoscopy conducted 2 months prior to surgery shows right vocal cord palsy. (B) No abnormal adduction of the vocal fold occurred during inspiration on transnasal fiberoptic laryngoscopy after removing continuous positive airway pressure.
The initial vital signs of patient in the operating room were systolic/diastolic blood pressure of 150/70 mmHg and oxygen saturation of 98% on room air. He had normal sinus rhythm on electrocardiography. General anesthesia was induced with 120 mg propofol and 40 mg rocuronium, followed by preoxygenation with 5 L oxygen. Two min after injecting the rocuronium, the patient was intubated with a 6.5 mm endotracheal laser tube (Medtronic, Laser-shield II 6.5 mm I.D. × 10.0 mm O.D.). The tube was fixed at 22 cm on a maxillary incisor. Eight ml of methylene blue solution was administered for ballooning. Anesthesia was maintained with 6−7 vol% desflurane in an oxygen-air mixture under a 0.3 fraction of inspired oxygen (FIO2). Total gas flow was 4 L/min. At the end of the surgical procedure, 15 mg pyridostigmine and 0.4 mg glycopyrrolate were administered after irregular spontaneous breathing of the patient. Five min after administering the anticholineasterase, we removed the tube after confirming clear consciousness of the patient and inspiratory pressure less than −40 mmHg. The patient responded well to commands and 5 sec grip strength was good on both hands. The patient complained of dyspnea and inspiratory stridor immediately after extubation. Oxygen saturation was maintained > 97% by pulse oximetry at FIO2 1.0. Clear lung sounds were heard throughout both lung fields on auscultation. The patient was placed on a face mask for manual ventilation and inhaled racemic salbutamol sulfate (100 µg as salbutamol) twice through the face mask to facilitate bronchodilation. We administered 140 mg sugammadex to completely reverse the effect of the muscular relaxants. However, the patient continued to complain of dyspnea and inspiratory stridor.
A transnasal fiberoptic laryngoscopy was done in the operating room. Laryngeal edema, right vocal cord palsy, and paradoxical adduction of the left vocal cord during inspiration were detected by laryngoscopy. Hydrocortisone sodium succinate (100 mg) was administered intravenously to alleviate the laryngeal edema and 1 mg midazolam was administered intravenously twice for anxiolysis. The patient still complained of dyspnea and inspiratory stridor after 5 min so we applied 5 cmH2O manual ventilation by face mask to support the patient's self-ventilation and the dyspnea and inspiratory stridor improved immediately. Therefore, we applied 5 cmH2O CPAP via a face mask (Fig. 2). Immediately after applying CPAP, the dyspnea and inspiratory stridor were relieved. A second transnasal fiberoptic laryngoscopy was performed 30 min after applying CPAP. We detected left vocal fold abduction from the midline on inspiration and widening of the glottic gap on the exam. The patient's dyspnea and inspiratory stridor recurred immediately after CPAP was removed. We decided to maintain 5 cmH2O CPAP applied via a face mask during transport to the intensive care unit (ICU). An arterial blood gas analysis (ABGA) was done immediately in the ICU and showed pH 7.4, PaCO2 39.8 mmHg, PaO2 104.2 mmHg and SaO2 97.7%. He had maintained oxygen saturation > 95% on a high-flow nasal oxygen cannula system (Optiflow™, Fisher & Paykel Healthcare Limited, Auckland, New Zealand) under settings of FIO2 0.35 and flow 35 L/min (Fig. 3). Nasopharyngeal pressure was about 2 cmH2O [3]. Another transnasal fiberoptic laryngoscopy was performed 9 hours after extubation and no left vocal fold adduction movement was detected on inspiration. The patient stopped complaining of dyspnea and inspiratory stridor. The high-flow nasal oxygen cannula system was removed 31 hours after extubation. The results of an ABGA at that time were pH 7.411, PaCO2 43.0 mmHg, PaO2 65.6 mmHg, and SaO2 93.6%. No left vocal fold adduction movement was detected on inspiration during transnasal fiberoptic laryngoscopy (Fig. 1B).
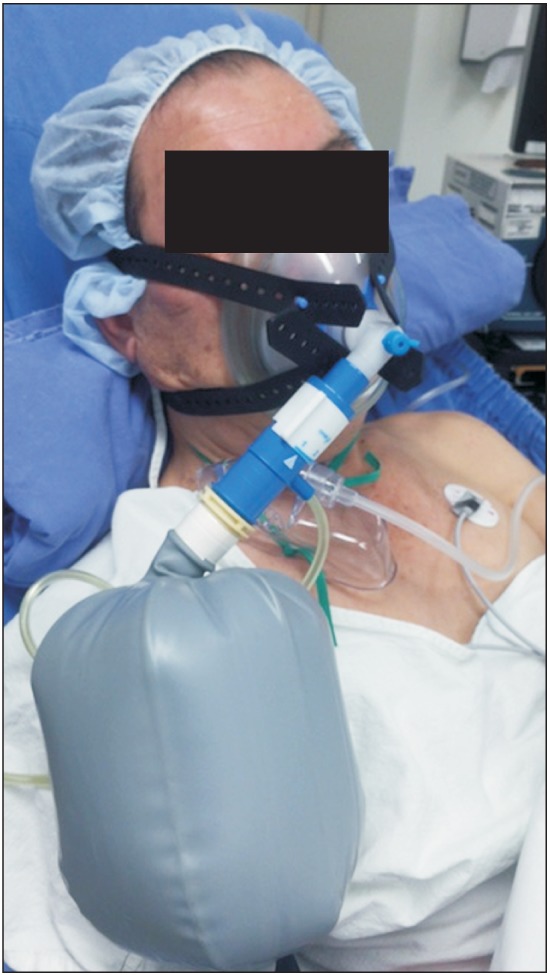
Continuous positive airway pressure of 5 cmH2O was applied to the patient via face mask in the post-anesthesia care unit.
Discussion
PVFM is a disorder that presents paradoxical adduction of the vocal fold during inspiration and abduction during expiration [2]. It was reported first by Patterson et al. [4] in 1974. At that time, PVFM was considered as neurologic or psychiatric disorder, therefore it was described as "Munchausen's stridor".
The symptoms of PVFM are inspiratory stridor, shortness of breath, choking sensation, voice changes, and cough due to the limited airway opening associated with paradoxical vocal fold adduction [2]. The major differential diagnoses are asthma, bilateral vocal cord paralysis, unilateral vocal cord paralysis, vocal cord granuloma, subglottic or glottis stenosis, laryngomalacia, tracheomalacia, benign or malignant neoplasm of the upper airway, and palatine or lingual tonsil hypertrophy. PVFM is often misdiagnosed as asthma because its clinical symptoms are similar with those of asthma. Patients with PVFM are resistant to asthmatic medical therapy, therefore, they tend to be exposed to unnecessary long-term steroid therapy and escalated drug use. Features that help distinguish PVFM from asthma are inspiratory stridor heard loudly over the larynx, rare sputum production, and associated voice changes. Chest auscultation is good diagnostic method because laryngeal sounds can echo through the thorax [5].
The etiology of PVFM remains uncertain. PVFM mainly affects young women with anxiety or an emotional disorder [6]. The organic causes of PVFM are brain stem compression, upper or lower motor neuron injury, and a movement disorder similar to Parkinson's disease [1]. The functional causes of PVFM include conversion disorder, anxiety disorder, depression, personality disorder, and stress disorder [7]. According to a 2002 study by Forrest et al. [5], psychologically based PVFM accounts for 70% of all PVFM cases. Patients with psychologically based PVFM present with conversion disorder alone or accompanying diseases, such as asthma, gastroesophageal reflux disease, laryngeal sicca, chronic laryngitis, and laryngeal sensory neuropathy.
Malingering patients are seen infrequently. The remaining 30% are patients with laryngeal hyperreactivity disorder or neurologic factors. Neurologic factors consist of focal respiratory dystonia with or without spasmodic dysphonia, multiple sclerosis, and autonomic dysfunction [5].
The gold standard for diagnosing PVFM is direct visualization of paradoxical inspiratory adduction of the vocal fold by laryngoscopy while the patient is complaining of symptoms, such as inspiratory stridor and dyspnea. Transnasal fiberoptic laryngoscopy is an excellent diagnostic method for PVFM. A pulmonary function test (PFT) can be helpful when there is a cut-off or flattening of the inspiratory limb on spirometry that suggests an extrathoracic obstruction. Clinical symptoms and laryngoscopic findings are essential for diagnosing PVFM but a PFT is not [8].
Typical findings on transnasal fiberoptic laryngoscopy are adduction of all parts of the true vocal cord and posterior diamond-shaped glottal chinking on inspiration. Adduction of vocal fold may also be seen on expiratory cycle. If the patient is asymptomatic at the time of laryngoscopy, some specific examination, such as metacholine challenge, exercise, histamine, and pungent smell, should be performed to trigger PVFM [2].
Treatments for PVFM include reassurance, speech therapy, psychotherapy, biofeedback, benzodiazepines, and inhaled anticholinergics. Heliox (helium-oxygen mixture) and botulinium toxin therapy also are available. If the patient does not improve with these therapies, positive pressure ventilation, a bag-valve-mask, or CPAP should be considered.
In an emergency situation, such as postoperative PVFM, an adequate oxygen supply and manual ventilation should be done first for emergency management. Invasive procedures, such as endotracheal intubation or tracheostomy, are performed under a falling oxygen saturation condition due to near complete vocal cord obstruction [9].
In this case, the patient was self-medicating with a benzodiazepine preoperatively because of concern about the right hand tremor. We administered 2 mg midazolam intravenously for anxiolysis. However, his symptoms did not improve. The SpO2 of the patient was maintained > 97% after extubation, therefore, we decided to apply CPAP rather than performing invasive procedures, such as re-intubation or a tracheostomy. Applying CPAP in a patient with PVFM lowers expiratory flow and increases lung volume, which helps the glottis to open and relieves dyspnea [10]. CPAP also reduces the effort needed for inspiration by establishing a favorable pressure gradient for inhalation [2].
Patients with PVFM generally have a good prognosis without long-term sequelae [11]. However, few patients with PVFM encounter an emergency situation, such as severe dyspnea. Therefore, rapid diagnosis and treatment are very important for a patient's prognosis.
PVFM is very difficult for anesthesiologists to diagnose in the post-anesthesia care unit because the gold standard for PVFM is transnasal fiberoptic laryngoscopy. However, PVFM should be considered when patients complain of dyspnea and stridor after endotracheal extubation. In conclusion, oxygen supply and positive pressure ventilation could be applied first in a case of PVFM when the patient complains of severe inspiratory dyspnea after endotracheal extubation. If the patient continues to suffer from prolonged dyspnea after supplying oxygen and positive pressure ventilation, CPAP could be effective before performing invasive procedures, such as endotracheal intubation or a tracheostomy.