Emergency cesarean section in an epidemic of the middle east respiratory syndrome: a case report
Article information
Abstract
Only a few reports have been published on women with an infectious respiratory viral pathogen, such as Middle East Respiratory Syndrome (MERS) Coronavirus delivering a baby. A laboratory confirmed case of MERS was reported during a MERS outbreak in the Republic of Korea in a woman at gestational week 35 + 4. She recovered, and delivered a healthy baby by emergency cesarean section (C-sec). We present the clinical course and the emergency C-sec in a pregnant woman with MERS.
A novel coronavirus that had not been seen previously in humans was identified for the first time in late 2012 in a resident of the Middle East now known as the Middle East Respiratory Syndrome Coronavirus (MERS-CoV) [1]. The first laboratory-confirmed case of MERS-CoV was reported in the Republic of Korea on May 20, 2015. MERS-CoV has caused about 186 infections in the Republic of Korea, including one pregnant patient. Most cases were transmitted in hospitals via respiratory droplets but the modes of transmission are incompletely defined [2].
MERS-CoV is a coronavirus known to cause acute respiratory illness-associated fever, which can progress rapidly to respiratory failure and renal failure in some patients. However, additional factors must be considered in pregnant patients. Pregnant women are traditionally considered a high risk group for viral infection, such as MERS, due to changes in their immune responses and the fetal effects of a severe respiratory syndrome [3]. A delivering a baby involves large loses of blood, amniotic fluid, and other body fluids, medical workers must take precautions against infection. However, only a few studies and case reports are available on confirmed cases of MERS and pregnancy [4]. A few cases of patients with other infective respiratory viral pathogens delivering a baby have been reported. Thus, it is very difficult to draw conclusions on the effects and management of MERS-CoV in pregnant women. We report a case of MERS in a pregnant woman with additional details on the clinical course and an emergency cesarean section (C-sec).
Case Report
A 39-year-old woman (gravida 2 para 1) had reversetranscription polymerase chain reaction (RT-PCR)-confirmed MERS-CoV at 35 + 4 weeks of gestation. This patient had gestational-related diabetes, but it was well-controlled by diet without medication. She was admitted to obstetrics ward of our institution on April 19 due to premature labor. During the 6-week admission period, she visited her mother who was hospitalized in the emergency department of the same institution on May 27 and 28, which was the same time that a known MERS-CoV super-spreader patient was treated there. Her mother developed illness with fever on day 7 after contacting the super-spreader and was confirmed positive by MERS-CoV RT-PCR.
Our case complained of severe myalgia without fever on June 8 and was admitted to the isolation ward 5 days after discharge. Her sputum sample was positive for MERS-CoV by RT-PCR. Creactive protein (CRP) was elevated to 1.95 mg/dl and a chest X-ray revealed patchy opacity in both lower lobes, suggesting bronchopneumonia (Fig. 1). A chest X-ray taken 4 days later was improved and CRP had decreased to a value within the normal range (0.15 mg/dl). However, she complained persistently of episodes of dyspnea, sputum, and myalgia without fever. She was provided supportive treatment, but no antiviral agent or steroid because the disease was not progressing. Fetal growth was within normal gestational limits, and she recovered gradually from the myalgia and other symptoms. The results of RT-PCR follow-up tests performed on June 19 and 21 were all negative in sputum samples. The Division of Infectious Diseases confirmed a full recovery, and she moved to the obstetrics ward on June 22 where she was expected to have a normal delivery. However, she began sudden bleeding with abdominal pain on the day after recovery was declared. A pelvic examination with a speculum confirmed the vaginal bleeding. However, the transabdominal ultrasonography did not detect any placental problems or hidden hemorrhaging. Baseline fetal heart rate (FHR) was 120–130 bpm with moderate variability. An obstetrician diagnosed placental abruption based on the symptoms and physical examinations. We considered spread of MERS-CoV because her symptom onset was very recent, and delivery would involve a large volume of contagious body fluids. An emergency C-sec under combined spinal-epidural anesthesia (CSE) was scheduled with precautions. The surgery was performed in a designated negative pressure-ventilated isolation operating room. All designated specialized infection control personnel prepared for the surgery and wore enhanced personal protective equipment (PPE) (Fig. 2).
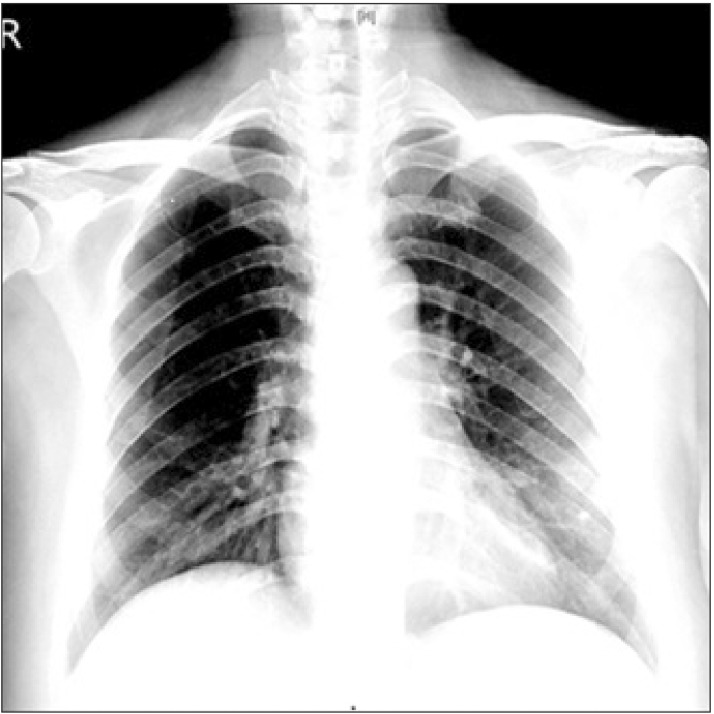
Chest X-ray performed on day 2 of hospital admission, showing patchy opacity in both lower lung lobes.

All medical staff used enhanced personal protective equipment, including an N95 mask, surgical cap, double gown, double gloves, shoe covers, and a powered air-purifying respirator during operative procedures.
A medical worker with PPE transferred the patient on an exclusive elevator. She was very anxious in the operating room, so we provided emotional support but no premedication. She was 167 cm tall and weighed 79 kg (body mass index, 28.33 kg/m2). Her baseline blood pressure and heart rate were 120/50 mmHg and 82 beats/min, respectively. SpO2 was 100% on room air and electrocardiograph results showed normal sinus rhythm. The patient was placed in the right lateral decubitus position, and epidural anesthesia was attempted with a 17-gauge Tuohy needle via the median approach at L3–4. The epidural space was detected using the loss-of-resistance technique, and a 22-gauge epidural catheter was inserted (Fig. 3). A lumbar puncture was performed with a 25-gauge Whitacre spinal needle at the L4–5 interspace, and 6 mg of 0.2% ropivacaine and 20 µg fentanyl were injected intrathecally. Levobupivacaine (0.25%, 10 ml) was administered through the epidural catheter. The patient was placed in the supine position with a left lateral tilt to avoid aortocaval compression. Anesthesia was assessed bilaterally using cold and light touch, and the spinal injection produced a T4 block after 10 min. The C-sec was performed uneventfully without pain or discomfort. The baby was 3.14 kg, with Apgar scores at 1 and 5 min of 9 and 10. Umbilical vein pH was 7.363. A 10 × 10 cm blood clot was extracted and approximately 20% of the placenta appeared to be abrupted.

Vision and movement are limited with personal protective equipment. An experienced senior anesthesiologist performed the combined spinal-epidural anesthesia.
Total operation time was 150 min, with an estimated blood loss of 700 ml. A total of 2700 ml of crystalloid solution and 1000 ml of colloid were administered during the surgery. An infusion of oxytocin (10 units/1000 ml Ringer's lactate solution) and carbetocin (1000 µg/10 ml normal saline) was administered to produce uterine contractions and minimize blood loss, respectively.
She was administered in the operating room until a recovery block to T8, then the patient was transferred directly to a single room on the obstetrics ward. The baby was isolated in the intensive care unit, and a MERS-CoV test was negative. Maternal serum and the placenta were MERS-CoV negative. Breastfeeding was allowed by pumping instead of sucking. She was discharged on postoperative day 7. The mother and baby are doing well.
Discussion
We performed an emergency C-sec in a patient with a highly infectious respiratory viral pathogenic disease. Sudden placental abruption occurred at midnight, and an immediate C-sec was recommended [5]. Our multidisciplinary team had discussed the delivery and had developed emergency scenarios; thus, the C-sec progressed without delay under the regional anesthesia (RA) for several reasons. She was not actively bleeding, and only moderate variability in FHR was detected without deceleration, so we had ample time to perform the CSE. Although the decision-to-deliver interval for general anesthesia (GA) is faster than that for RA [6], RA is preferred to GA because it is safer and causes less maternal and neonatal morbidity than those of GA [67]. The other consideration in this case was spread of the infection. Although her chest X-ray and laboratory results improved, the patient had pneumonia within 2 weeks. We were not convinced that the lung parenchyma had fully recovered, and tracheal intubation/extubation in a patient with MERS could expose the medical staff to a high aerosolized viral load. Thus, the C-sec was performed by an experienced senior anesthesiologist. Potentially high-risk procedures should be performed by experienced medical staff during an infectious disease outbreak period [8].
MERS is thought to be transmitted mainly via respiratory droplets and close human contact, but the mode is not completely defined. Although we already had some infection-control protocols applicable for the operating room for known infectious diseases, such as pulmonary tuberculosis, viral hepatitis, human immune deficiency virus, and antibiotics-resistance bacterial infection before the MERS outbreak, we developed a new operating room protocol for patients with confirmed or suspected MERS because of the high infectivity and unknown transmission mode.
The patient's sputum was MERS-CoV negative based on RTPCR before delivery, so it is unclear if strict precautions were necessary [9]. However, the patient had been declared recovered for only 1 day, and illness onset was within 2 weeks. In addition, a C-sec frequently involves large volumes of blood, amniotic fluid, and other body fluids, and these could be infection sources. No other case reports are available on delivering a baby for a women with MERS, but several cases of labor and delivery in women with severe acute respiratory syndrome (SARS) have been published [1011]. Viral shedding from maternal body secretions, stool, cerebrospinal fluid, and peritoneal fluid has been reported in SARS cases, suggesting that MERS-CoV could be present in any body fluid and transmitted during C-sec, which is why we treated this patient for confirmed MERS perioperatively. We followed our institution protocol for patients with MERS and referred to the SARS obstetrics guidelines. According to the management guidelines for obstetric patients with SARS [12], all deliveries should be managed in a designated negative-pressure isolation room and by designated personnel with specialized infection control PPE.
As we have no permanent negative pressure operating rooms at our institution, two operating rooms were converted to negative-pressure [13]. These rooms were connected, but had separate air-conditioning and humidification units with individual atmospheric air inlet and exhaust systems. We used one as the operating theater and the other as a PPE changing room. The air pressure in these rooms was maintained at -4.7 Pa (operating theater) and -1.2 Pa (changing room). An airflow visualization (smoke) test was carried out to ensure negative pressure. Modifications were made to minimize outflow of contaminated air from the MERS-related operating rooms. Airflow in the operating room was > 14 air exchanges per hour. All anesthetic and surgical team staff used enhanced PPE comprised of a N95 mask, surgical cap, double gowns, double gloves, shoe covers, and powered air-purifying respirators. After the patient recovered from anesthesia in the operating room, the patient was transferred back to the ward by the same dedicated anesthesiologist who changed into new PPE to minimize MERS-CoV exposure. An N95 mask was fit on the patient during the transfer and Roberge et al. [14] reported that use of an N95 mask by a healthy women during late pregnancy did not change any physiological or subjective findings compared with those of healthy, non-pregnant women during sedentary activities and exercise for 1 hour. If a pregnant woman is infectious, they may be able to use an N95 mask without complications. Finally, the MERS-designated spaces were all ventilated for 100 min and thoroughly decontaminated after the case. All medical staff involved in the delivery remained healthy.
Placental abruption occurred in our case, which is one of most commonly encountered uteroplacental bleeding disorders with high rates of perinatal-maternal morbidity and mortality. Several reports have indicated that maternal acute and chronic respiratory diseases during pregnancy are associated with placental abruption [15]. Acute and chronic respiratory conditions may trigger an inflammatory and hypoxic process at the maternal-fetal interface that may lead to placental abruption. However, placental abruption occurs in only about 1% of pregnancies [15]. Only two case reports are available on pregnant women with MERS who delivered a baby. A woman in Abu Dhabi died after giving birth to a baby by C-sec, and the other case was associated with a stillbirth in Jordan [4]. Thus, we were not convinced that the placental abruption was related with MERS. Our patient was complicated by a history of premature labor, gestational diabetes, and advanced maternal age. However, the pregnancy had a favorable outcome for the mother and baby. The reasons for the good outcome are unclear but may be due to the late stage of gestation and earlier detection of MERS. Long-term follow-ups of infants and mothers are needed to fully define the pregnancy-related risks of MERS infection.
Most human infections result from human-to-human spread, and when particular medical procedures are combined with poor infection control the virus can disseminate within the hospital. Thus, protocols and education for medical workers are essential to minimize exposure and protect against transmitting the pathogen during an infectious disease outbreak. Surgeries, particularly those performed in an emergency situation, have a high risk for spreading a contagious pathogen. Thus, a multidisciplinary team of emergency personnel should be prepared, including surgeons, physicians, nurses, and anesthesiologists. Early and strict infection control strategies and clinical guidelines for infectious patients, such as those with MERS, should be established.
