Anesthetic experience of patient with isolated left ventricular noncompaction: a case report
Article information
Abstract
Isolated left ventricular noncompaction (LVNC) is a rare primary genetic cardiomyopathy characterized by prominent trabeculation of the left ventricular wall and intertrabecular recesses. Perioperative management of the patient with LVNC might be challenging due to the clinical symptoms of heart failure, systemic thromboembolic events, and fatal left ventricular arrhythmias. We conducted real time intraoperative transesophageal echocardiography in a patient with LVNC undergoing general anesthesia for ovarian cystectomy.
Isolated left ventricular noncompaction (LVNC), also called spongiform cardiomyopathy is a rare cause of heart failure. Arrest of compaction process during normal heart development is thought to be the cause of LVNC [12]. Echocardiographic demonstration of the apical trabeculation of left ventricle and blood flow from ventricular cavity into the intertrabecular recesses are the main pathologic findings in the diagnosis of LVNC [345]. In patients with LVNC, mechanical dyssynchrony between non-compacted and compacted myocardium results in global left ventricular (LV) dysfunction [6] and leads to the clinical symptoms of heart failure. Other important clinical presentations of LVNC include thromboembolic events and arrhythmias due to microcirculatory dysfunction in the noncompacted myocardium and stasis of blood in the recesses [1]. In addition, left ventricular hypertrabeculation is observed in association with a variety of neuromuscular disease (NMD) [7]. Therefore, the anesthetic management of these patients requires special attention to the cardiac function and adverse reaction to general anesthesia. Perioperative echocardiography might be the method of choice to access cardiac function and thromboembolism during general anesthesia in such patients.
We reported our anesthetic experience with a LVNC patient who underwent laparoscopic ovarian cystectomy under general anesthesia with real time transesophageal echocardiography.
Case Report
A 61-year-old woman with a left ovarian cyst was scheduled for laparoscopic ovarian cystectomy under general anesthesia. At age 49, she had difficulty going up 3 stairs and started a dry cough. She was referred to our institution for further evaluation and treatment. She had no other disease including NMD. A transthoracic echocardiogram (TTE) revealed decreased LV cavity size and mild to moderate LV systolic dysfunction with an ejection fraction (EF) of 40% by visual estimation. The patient's LV walls, especially apical and interventricular septum (IVS), were markedly thickened, and showed abnormal relaxation that was compatible with noncompaction of LV. She was started on digitalis, diuretics and anticoagulants. Recently, her symptoms were aggravated. She was able to perform basic activities of daily life, but frequently felt chest discomfort. On physical examination, she showed regular heart beats with grade II systolic murmur. Electrocardiogram (ECG) showed sinus bradycardia with 1st degree atrioventricular block, multiple ventricular premature contractions and left bundle branch block. TTE examination demonstrated normal LV cavity size, moderate to severe LV systolic dysfunction with EF of 30% by visual estimation and diastolic dysfunction with left atrial enlargement. Consistent with noncompaction, TTE showed prominent trabeculation with deep intertrabecular recesses and increased velocity at apical trabeculation (Vmax = 3.02 m/sec, Peak pressure gradient = 36.4 mmHg) (Fig. 1).

(A) Preoperative transthoracic echocardiography (TTE) shows the prominent trabeculation of left ventricle (LV). Arrows indicate the trabeculations. (B) Preoperative TTE reveals blood flow from LV cavity into the intertrabecular reccesses, as visualized by color flow Doppler.
We planned general anesthesia with real time TEE monitoring to access intraoperative cardiac function. We chose TEE, without placement of a pulmonary artery catheter (PAC), because we were concerned with the increased risk of life threatening complications associated with the use of PACs, such as dysrhythmias [8]. No premedication was administered to the patient. Continuous monitoring of blood pressure was initiated after placement of an arterial catheter at the right radial artery under local anesthesia. Anesthesia was induced with etomidate 0.25 mg/kg, midazolam 2 mg and continuous infusion of remifentanil under 100% O2 mask ventilation, followed by the muscle relaxant cisatracurium 0.15 mg/kg to facilitate tracheal intubation. Patient's lungs were ventilated at a rate of 10 breaths/min with an oxygen/air mixture using positive pressure ventilation. Anesthesia was maintained with continuous infusion of propofol and remifentanil, and standard monitoring of invasive arterial blood pressure, ECG, peripheral oxygen saturation (SpO2), bispectral index (BIS), train-of-four (TOF), and TEE.
Intraoperative TEE confirmed the preoperative TTE findings. TEE examination showed aneurysmal change of apex, trabeculation in anterior and anterolateral wall of LV, increased velocity at apical intertrabeculation and intertrabecular recesses (Fig. 2).
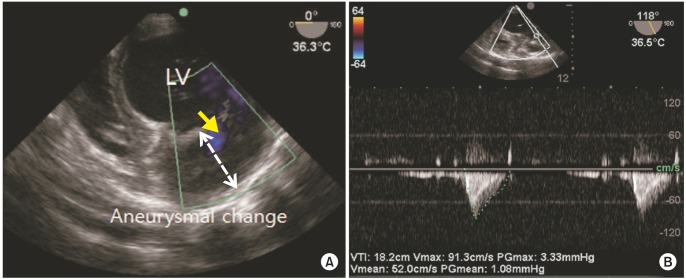
(A) Intraoperative transesophageal echocardiography (TEE) shows prominent trabeculation with aneurysmal change. (B) Intraoperative TEE reveals increased velocity at apical intertrabeculation.
Immediately after anesthetic induction, the patient's systolic blood pressure (SBP) abruptly decreased from 120 mmHg to 85 mmHg and heart rate (HR) was also decreased from 71 bpm to 45 bpm. The real time TEE examination demonstrated mild to moderate MR, global hypokinesia, visually estimated EF of 30%, and calculated cardiac output by TEE of 1.8 L/min/m2. Continuous infusion of the inotropic agent dobutamine was started at 3 µg/kg/min to increase cardiac output. SBP and HR were maintained during the entire operation within the range of 90 to 140 mmHg and 51 to 70 bpm, respectively. There were no significant hemodynamic changes during insufflation and desufflation of carbon dioxide.
At the end of surgery, all the continuous anesthetic medications were tapered off. Soon after, TOF monitor reached 4/4 twitches, but BIS still ranged from 50 to 60 for about 50 minutes. Apnea, pinpoint pupils and lower range of BIS suggested opioid effects. Finally, naloxone, a µ-receptor antagonist that competitively inhibits opioids side effects, was administered intravenously at 120 µg. Immediately after the injection, she opened her eyes on a verbal command and tidal volume recovered to 250−300 ml. The total operation time was 120 minutes but, the total anesthesia time was 214 minutes because of delayed awakening. Total infused amounts of crystalloid were 700 ml and estimated blood loss and urine output were 100 ml and 90 ml, respectively.
The patient was transferred to the intensive care unit. At postoperative day 1, she was transferred to the general ward uneventfully. The patient was discharged from the hospital on postoperative day 6 and she had regular follow up in the cardiology and obstetric departments.
Discussion
LVNC is defined as thickened LV segments with a distinct 2-layer appearance and prominent trabecular meshwork of the apex and adjoining inferior and lateral walls [39]. Often the affected myocardial segments are hypocontractile and display a 2-layer appearance with a thicker, 'non-compacted' or hypertrabeculated layer on the epicardial side [10]. LVNC is an uncommon condition; however, the estimated prevalence may be higher because asymptomatic individuals rarely undergo imaging studies and the condition likely remains undetected. Stanton et al. [11] detected isolated LVNC in 0.02% of 141,047 patients who were at least 16 years of age. However, increased awareness and improvements in imaging resolution may also contribute to an increase in the number of reported cases.
Diagnosis of LVNC is based on the echocardiographic findings of at least 4 prominent trabeculation, deep intertrabecular recesses and blood flow from the ventricular cavity into the intertrabecular recesses as visualized by color flow Doppler [9].
The clinical presentation of patients with LVNC is variable including congestive heart failure, arrhythmias and systemic thromboembolic events. The current recommendations for treatment according to the international guidelines of heart failure management include beta blocker, angiotensin - converting - enzyme inhibitor and diuretics [12]. Some clinicians prescribe anticoagulants for LVNC patients who develop atrial fibrillation to reduce the risk of thromboembolism [10]. Patients with LVNC may require insertion of ventricular assist devices or cardiac transplantation [1013]. In a study on 34 patients, 12% required transplant and 35% died at 44 months follow up [4]. ECG reveals abnormalities in a majority of patients with LVNC. Most commonly, LV hypertrophy or ST-T wave changes are noted in the ECG. However, more severely, atrial fibrillation, Wolff-Parkinson-White syndrome and other ventricular arrhythmias have also been described [2]. In a study by Fischer et al. [14], the patients were followed up for a mean of 30 months. During this period, 10 deaths were reported of which 60% occurred as a sudden event, whereas 34% of patients required hospitalization for symptoms of heart failure. Ventricular tachycardia was seen in 36% of patients and thromboembolic events in 9%. A familial occurrence was reported in 33% of patients.
Although classified by the American heart association as a primary cardiomyopathy, LVNC has been described in association with extracardiac conditions, most notably NMD [7]. The exact incidence is unknown, but in one study, 80% of patients with LVNC were diagnosed with an NMD [9]. A number of genes are thought to be associated with this condition.
The anesthetic management of these patients requires specific attention to heart failure, arrhythmia, thromboembolic events and NMDs [210]. TEE is a useful monitor to guide intraoperative anesthetic and fluid management in the presence of severely depressed LV and RV function [4]. Real time TEE might be the method of choice to access cardiac function and thromboembolism during general anesthesia in these patients. When the echocardiography is not suitable, non-invasive cardiac output monitoring devices might be helpful in the evaluation of cardiac function. Also patients with LVNC are susceptible to develop severe arrhythmia. Stressors that increase sympathetic tone and anesthetic drugs can provoke severe arrhythmia including fatal ventricular fibrillations. In addition, the dose of non-depolarizing neuromuscular blocking agents should be titrated under continuous monitoring of TOF [15].
In conclusion, echocardiography is the method of choice in the diagnosis of LVNC, a rare cardiomyopathy. Anesthesiologist should be specifically concerned with the presenting symptoms of LVNC, heart failure, thromboembolic events, arrhythmia and NMDs.
To the best of our knowledge, this is a rare case report of LVNC under general anesthesia reported by a Korean anesthesiologist. This case adds to the understanding of anesthetic management of LVNC patients using TEE. Further studies on the disease and its anesthetic management are required.