 |
 |
|
|
Abstract
Spinal cord stimulation (SCS) in trials involving external stimulation are easily conducted under local anesthesia. However, implantation of a permanent SCS system is painful, and can be intolerable in some patients. Epidural anesthesia can be used to perform the SCS implantation without discomfort if the patient can localize the area of paresthesia. However, little is known about epidural anesthesia for SCS. This paper reports 23 cases of permanent SCS with a cylindrical type lead implanted under the epidural anesthesia. Epidural anesthesia was sufficient in 22 patients without discomfort and significant complications. The remaining patient experienced incomplete epidural anesthesia and required additional analgesics to blunt the pain. All the leads were placed consistent with the patient's report of paresthesia area under epidural anesthesia. Thus, epidural anesthesia is an effective and safe method for the optimal placement of SCS to minimize the discomfort for patients without impairing patients' response to the intraoperative stimulation test.
Spinal cord stimulation (SCS) is popular for the treatment of a variety of chronic neuropathic pain syndromes, including failed back surgery syndrome, complex regional pain syndrome, peripheral vascular disease and angina pectoris [1,2]. SCS is usually performed in two stages, with an external stimulator used first, followed by permanent implantation. The trials can be easily conducted by placing a temporary percutaneous epidural lead under the local anesthesia [3]. Permanent implantation is a surgical procedure that is commonly performed under the local anesthesia to enable intraoperative testing of paresthesia corresponding to the painful area [4]. Some patients find this method intolerable due to pain. Because the placement of SCS under general anesthesia precludes the use of intraoperative test stimulation, general anesthesia is not recommended [5].
Epidural anesthesia can be used to perform the SCS without discomfort if a cooperative patient can localize the area of paresthesia. However, not much is known about epidural anesthesia for SCS with a cylindrical type lead. This paper reports 23 cases of epidural anesthesia for the permanent placement of SCS.
A total of 23 patients with chronic intractable lower limb pain who had permanent SCS with a cylindrical type under epidural anesthesia between November 2010 and December 2013 were included in the case series. The Institutional Review Board of our hospital approved the study protocol. All patients had previously undergone a successful SCS trial with percutaneous electrodes for more than 3 days. A successful trial of SCS was defined as pain reduction or the satisfaction rate Ōēź 50% with evidence of increased activities. The percutaneous trial leads were removed and discarded regardless of the success of the trial.
Written informed consent was obtained from each patient the day before the permanent lead and the generator was placed. Electrocardiogram, blood pressure and peripheral oxygen saturation were routinely monitored throughout the procedure in the operating room. The patient was placed in the lateral decubitus position with the spine flexed for epidural anesthesia. Two percent lidocaine was infiltrated around the needle puncture site prior to the epidural anesthesia, which was induced by epidural bolus injection at the L2-L3, L3-L4 or L4-L5 level with local anesthetic solution in volumes from 10 to 30 ml to achieve the sensory blockade of T10 or higher. The volume and dosage of local anesthetics for epidural anesthesia were decided arbitrarily by the discretion of the responsible anesthesiologist. The patient was then arranged in the supine position and checked for the anesthetic level using a blunt needle every 5 min. The patient was placed prone with a pillow under the abdomen to reduce lumbar lordosis after an appropriate sensory level of block was obtained.
After the sterile skin preparation and draping was performed, fluoroscopic anteroposterior (AP) imaging was used to identify and mark vertebral segments and pedicles of L2, L3 and L4 with a sterile marker. A 5 cm long longitudinal skin incision was made aligning the centers of L2, L3 or L4 pedicle after the incision site was checked. A 14 gauge Tuohy needle was used for the paramedian approach with C-arm fluoroscopic image. After ensuring that the needle was positioned in the epidural space, the guidewire was used to make insertion of the electrode easier. The octode electrode lead (Advanced Neuromodulation System, Planco, TX, USA) was carefully advanced through the needle to one vertebral level above to the target placement, and then the lead placement was determined by the patient response during intraoperative stimulation. Successful stimulation was defined as paresthesia development in the patient's pain distribution area as in the trial stimulation. Fluoroscopic images in both AP and lateral views were taken after determining the placement of the lead. If a second lead was required, the second epidural needle was inserted inferior to the first needle. The second lead was positioned after the first lead has been completely positioned. After stylet and the needle were removed under fluoroscopic guidance, the leads were meticulously anchored with a 2.0 nonabsorbable silk. Final fluoroscopic images in both AP and lateral views at the end of this process were taken to ensure that lead migration did not occur.
A small incision was made in the flank below rib 12 for the exit of the lead, and then a tunnel was made in the deep subcutaneous fat by using a tunneling tool from the back incision. The lead was pulled out towards the flank incision through the passing straw. After irrigation of the lumbar incision area with a mixed solution of normal saline and antibiotic, it was closed and dressed.
After the patient was placed in the left semilateral position, sterile skin preparation and draping were performed again for the implantation of the generator in the left abdomen. A transverse incision about 5 cm long was made along the previously marked line in the left lower abdominal region just below the umbilicus level and subcutaneous pocket was created caudal to the incision. It was tunneled subcutaneously between the abdominal pocket site and the flank incision site. The lead was pulled through the passing straw. The integrity of the system was checked and the generator was positioned. The pocket site was irrigated with a mixed solution of normal saline and antibiotic, and sutured. Appropriate dressings covered all the wounds. The SCS was turned on and tested again to ensure adequate coverage of all painful areas after the patient had been completely recovered from epidural anesthesia. SCS parameters were recorded and compared at the trial (T0), permanent implant (T1) and postoperative stimulation 3 days after surgery (T2). The pain intensity was evaluated with a visual analogue scale (VAS) of 0 to 10 (where 0 means nothing and 10 represents the worst condition imaginable) before and after the permanent SCS implantation.
Twenty-three patients underwent epidural anesthesia for permanent SCS implantation (Table 1). Epidural anesthesia was sufficient to perform the implantation of the lead and generator in 22 patients without interference with the intraoperative test stimulation. Incomplete epidural anesthesia requiring an additional dose of fentanyl 100 ug to control pain was experienced in one case.
There was an interval of 15.2 ┬▒ 6.7 days between the trial and permanent SCS implantation. The patients' clinical characteristics are presented in Tables 1 and 2. The average time of the permanent SCS implantation was 126.5 ┬▒ 41.4 min. The level of sensory block was not recorded in five patients. The highest level of the sensory block was the fourth thoracic segment (9.5%) and the lowest level was tenth thoracic segment (4.8%), which provided sufficient analgesia for all SCS procedures. The epidural local anesthetics included 2% lidocaine in three patients, 0.75% ropivacaine in one patient and a mixed solution of 2% lidocaine and 0.75% ropivacaine in 19 patients. The average volume of local anesthesia was 16.4 ┬▒ 4.3 ml (range 10-30). The incidence of hypotension was 43.5% and the incidence of bradycardia was 30.4%. Hypotension and bradycardia were treated well with volume expansion, ephedrine, phenylephrine or atropine. There were no serious complications of epidural anesthesia.
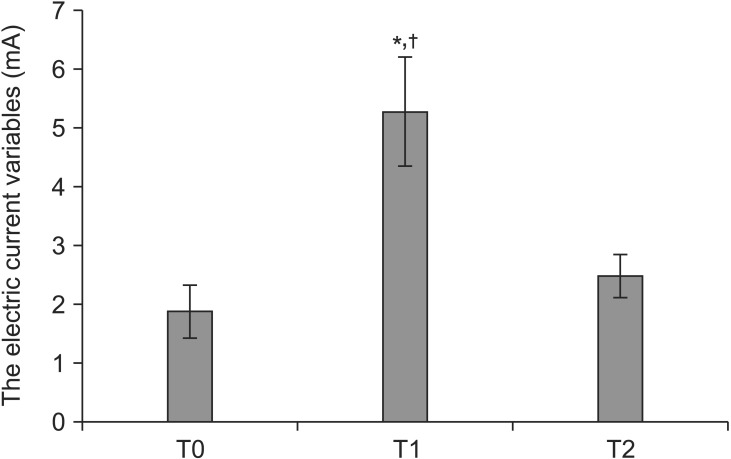
All the leads were placed according to the patient's report of paresthesia coverage when stimulations of spinal cord were created under the epidural anesthesia. The electric current variables (mA) needed to reproduce paresthesias at T0, T1 and T2 were 1.87 ┬▒ 1.02, 5.27 ┬▒ 2.13 and 2.47 ┬▒ 0.86, respectively. The electrical current intensity at T1 was significantly higher than those at T0 (P < 0.001) and T2 (P < 0.001) when compared with each other (Fig. 1). The frequency (Hz) at T0, T1 and T2 ranged from 30 to 60, and the pulse width (┬Ąs) at T0, T1 and T2 ranged from 200 to 300. All patients experienced pain improvement (Table 2) and the average pain intensity (VAS) scores were 8.6 ┬▒ 0.6 and 3.6 ┬▒ 0.6 before and after SCS implantation.
It has been generally considered that adequate paresthesias may not be obtained if the nerve roots or the spinal cord are blocked by epidural anesthesia. However, this report demonstrates that successful epidural anesthesia in SCS implantation without disturbing perception. During the procedures, epidural anesthesia provided a satisfactory analgesic effect, with the exception of one patient who felt pain, which was controlled by intravenous fentanyl 100 ug. Hypotension and bradycardia were the most common cardiovascular changes during the epidural anesthesia for SCS, requiring careful managements of blood pressure and heart rate.
SCS can be used for a variety of anesthetic techniques including local anesthesia, local anesthesia with sedation, spinal anesthesia, epidural anesthesia, monitored anesthesia care and general anesthesia [4]. SCS is generally performed under local anesthetic infiltration with or without sedation considering intraoperative test stimulation. Local anesthesia does not prevent the patients from suffering significant discomfort and stress, and occasionally some patients have to abandon the procedures [6]. Increased administration of sedatives and analgesics in the prone position may cause airway problems and respiratory depression [7]. Excessive sedation and general anesthesia prevent the patient from responding to the distribution of paresthesias for optimal positioning of the lead [8].
The findings of the present study correspond well with those of several previous studies. Garc├Ła-P├®rez et al. [5] reported that epidural anesthesia is suitable for identification of paresthesia in the optimal placement of the SCS leads. Zhang et al. [4] suggested that patient's perception of paresthesias during intraoperative testing is reliable, consistent and correlates well with the postoperative stimulation. Epidural anesthesia was an effective and safe method for the placement of SCS in those studies, because it allowed the physician to promptly observe the patients' perception of electrical stimulus over the range of areas with pain, followed by inducing pain relief.
The SCS devices used in this report have two distinct features from those of mentioned in two previous studies. Eight-channel cylindrical type electrodes with the constant current system were used in this report, whereas the two prior studies used paddle type leads with constant voltage system. Eight-channel cylindrical type electrodes are less invasive than the paddle type electrodes as they do not entail resection and removal of vertebral tissue [9]. However, the paddle type electrodes have better electrical conduction properties due to larger surface contact areas and unidirectional stimulating poles, and may be associated with a lower rate of migration as compared with cylindrical type electrodes [10,11]. Our cases demonstrate that the cylindrical type lead can also achieve sufficient paresthesia for the intraoperative stimulation under epidural anesthesia despite its weaker conductivity than the paddle type. The constant current SCS system provides a constant current flow with the change of voltage in response to impedance, while the constant voltage SCS system provides constant voltage and variable current according to impedance [12]. This is the first report on the epidural anesthesia for SCS implantation using cylindrical type leads with a constant current.
This report indicates the absence of a decline of paresthesia by epidural anesthesia, with no interference with the intraoperative stimulation test for the optimal placement of SCS leads. Although the stimulation intensity required to evoke optimal paresthesia was significantly higher than both trial tests and postoperative stimulation intensity in this report, adequate paresthesia was obtained in all patients under epidural anesthesia. This result was similar to previous reports, which proposed that the intraoperative voltage is significantly higher under epidural anesthesia because some of the superficial posterior column fibers may also be blocked by the anesthetic drugs diffusing [4,5]. The local anesthetics, which are deposited into the epidural space, primarily act on the nerve roots and then completely block both the sensory and motor transmission [13]. Additionally, the local anesthetics that diffuse into the subarachnoid space do not block the spinal cord completely and only partially block transmissions in the dorsal column [4,5]. Several studies reported that the spinal cord is not completely blocked by spinal anesthesia [6,14,15]. Intraoperative electrical stimulation test with an increased paresthesia threshold enabled positioning of the leads for SCS under epidural anesthesia. Because the analgesic effect was perfectly established under epidural anesthesia, patients were alert and cooperative to guide the optimal lead positioning, and it also facilitated tunneling of the lead and implantation of the pulse generator. We have experienced only minor complications, such as hypotension and bradycardia, with this anesthetic method of SCS.
In conclusion, this report shows that epidural anesthesia is an effective and safe method for the optimal placement of the SCS to minimize the discomfort for patients without impairing patients' response to the intraoperative stimulation test.
References
1. Jeon YH. Spinal cord stimulation in pain management: a review. Korean J Pain 2012; 25: 143-150. PMID: 22787543.



2. Ryu SW, Jeon HJ, Cho SS, Choi RM, Yoon JS, Ko HS, et al. Treatment of digit ulcers in a patient with Buerger's disease by using cervical spinal cord stimulation -a case report-. Korean J Anesthesiol 2013; 65: 167-171. PMID: 24024002.



3. Beems T, van Dongen RT. Minimally invasive placement of epidural plate electrodes under local anaesthesia in spinal cord stimulation. Acta Neurochir Suppl 2007; 97: 105-109. PMID: 17691364.


4. Zhang K, Bhatia S, Oh M, Whiting D. Epidural anesthesia for placement of spinal cord stimulators with paddle-type electrodes. Stereotact Funct Neurosurg 2009; 87: 292-296. PMID: 19590261.


5. Garc├Ła-P├®rez ML, Badenes R, Garcia-March G, Bordes V, Belda FJ. Epidural anesthesia for laminectomy lead placement in spinal cord stimulation. Anesth Analg 2007; 105: 1458-1461. PMID: 17959983.


6. Sarubbo S, Latini F, Tugnoli V, Quatrale R, Granieri E, Cavallo MA. Spinal anesthesia and minimal invasive laminotomy for paddle electrode placement in spinal cord stimulation: technical report and clinical results at long-term followup. Scientific World Journal 2012; 2012: 201053PMID: 22566761.




7. American Society of Anesthesiologists Task Force on Sedation and Analgesia by Non-Anesthesiologists. Practice guidelines for sedation and analgesia by non-anesthesiologists. Anesthesiology 2002; 96: 1004-1017. PMID: 11964611.


8. Mammis A, Mogilner AY. The use of intraoperative electrophysiology for the placement of spinal cord stimulator paddle leads under general anesthesia. Neurosurgery 2012; 70(2 Suppl Operative): 230-236. PMID: 21869720.



9. Villavicencio AT, Leveque JC, Rubin L, Bulsara K, Gorecki JP. Laminectomy versus percutaneous electrode placement for spinal cord stimulation. Neurosurgery 2000; 46: 399-405. PMID: 10690729.


10. North RB, Kidd DH, Olin JC, Sieracki JM. Spinal cord stimulation electrode design: prospective, randomized, controlled trial comparing percutaneous and laminectomy electrodes-part I: technical outcomes. Neurosurgery 2002; 51: 381-389. PMID: 12182776.


11. North RB, Kidd DH, Petrucci L, Dorsi MJ. Spinal cord stimulation electrode design: a prospective, randomized, controlled trial comparing percutaneous with laminectomy electrodes: part II-clinical outcomes. Neurosurgery 2005; 57: 990-996. PMID: 16284568.



12. Washburn S, Catlin R, Bethel K, Canlas B. Patient-perceived differences between constant current and constant voltage spinal cord stimulation systems. Neuromodulation 2014; 17: 28-35. PMID: 23837549.


13. Fink BR. Mechanism of differential epidural block. Anesth Analg 1986; 65: 325-329. PMID: 3954107.


14. Lang E, Erdmann K, Gerbershagen HU. High spinal anesthesia does not depress central nervous system function as measured by central conduction time and somatosensory evoked potentials. Anesth Analg 1990; 71: 176-180. PMID: 2375519.


15. Lind G, Meyerson BA, Winter J, Linderoth B. Implantation of laminotomy electrodes for spinal cord stimulation in spinal anesthesia with intraoperative dorsal column activation. Neurosurgery 2003; 53: 1150-1153. PMID: 14580282.



Fig.┬Ā1
The electric current variables (mA) at the trial (T0), permanent (T1) and postoperative stimulation 3 days after surgery (T2). There was a significant difference between T0 and T1, and T1 and T2. *P < 0.001 T0 vs. T1; ŌĆĀP < 0.001 T1 vs. T2.
Table┬Ā1
Characteristics of Patients and Permanent SCS Implantation
Table┬Ā2
Summary of Patient Charateristics and Permanent SCS
- TOOLS









