The usefulness of end-tidal carbon dioxide monitoring during apnea test in brain-dead patients
Article information
Abstract
Background
The apnea test (AT) is essential to confirming the diagnosis of brain death, but critical complications can occur if the AT is maintained over a long period. To minimize the AT period, we used end-tidal carbon dioxide (ETCO2) monitoring because ETCO2 is closely correlated with partial pressure of arterial carbon dioxide (PaCO2). The aim of the present study is to evaluate the usefulness of ETCO2 monitoring during apnea testing.
Methods
We reviewed 61 patients who were pronounced brain dead at our hospital from July 2009 to December 2012. The subjects were divided into two groups: the N-group, in which capnography was not used, and the C-group, in which capnography was used to monitor ETCO2. In the C-group, whenever arterial blood was sampled, the PaCO2 - ETCO2 gradients were calculated and the ventilator setting adjusted to maintain normocapnia prior to apnea testing.
Results
Twenty-eight subjects in the N-group and twenty-nine subjects in the C-group were included. The gender ratio, age, and cause of brain death were not different between the two groups. Prior to the AT, the normocapnia ratio was higher in the C-group than in the N-group. During the AT, the total test period was shorter in the C-group. Moreover, systolic blood pressure increased in the C-group and decreased in the N-group during apnea testing.
Conclusions
ETCO2 monitoring during AT allows the PaCO2 level to be predicted, which reduces the duration of the test and stabilizes systolic blood pressure. Thus, with ETCO2 monitoring, the AT can be fast and safe.
Introduction
Brain death is the irreversible loss of all brain functions, including the brain stem, caused by organic brain lesions [1,2]. Although the clinical criteria of brain death differ somewhat across countries, the apnea test is a mandatory step in many countries, including the U.S., the U.K., and Japan. The apnea test is undertaken to demonstrate the absence of spontaneous breathing when the partial pressure of arterial carbon dioxide (PaCO2) reaches a target level for sufficient stimulation of the respiratory center. However, complications such as respiratory acidosis, hypotension, hypoxia, and cardiac arrhythmia may occur if the confirmation of target PaCO2 takes a long time [3,4,5]. These complications can cause insufficient organ perfusion and increase the failure rate of organ procurement [6,7]. Therefore, it is important to minimize the performance time of apnea testing.
The American Academy of Neurology (AAN) established prerequisites to reduce the worsening of donor condition during the apnea test. One prerequisite is to maintain normocapnia (PaCO2 35-45 mmHg) prior to apnea testing. This is intended to prevent respiratory acidosis or alkalosis and minimize complications due to abnormal PaCO2. To maintain normocapnia and confirm the target level of PaCO2, serial arterial blood gas analysis (ABGA) is necessary.
We planned to use end-tidal carbon dioxide (ETCO2) monitoring to decrease the frequency of ABGA and the performance time of apnea testing because ETCO2 is closely correlated with PaCO2. With continuous monitoring of ETCO2 by capnography, we are able to predict PaCO2 indirectly [8,9,10,11] and control PaCO2 without repeated ABGA. Based on these assumptions, the intensive care unit team in our hospital furnished capnography and has been using capnography for deceased donors since November 2011. Therefore, in this study, we investigated the usefulness of capnography during the apnea test. The aim of the present study is to evaluate whether ETCO2 monitoring reduces the performance time and complications during the apnea test.
Materials and Methods
After obtaining the approval of the Institutional Review Board of our hospital, we investigated patients who were declared brain dead for organ donation from July 2009 to December 2012. We collected computerized and manual medical records of 61 patients from 14 to 75 years old.
After arrival in the intensive care unit (ICU), all subjects were managed under the protocol of the ICU, and vital signs (blood pressure, pulse rate, and oxygen saturation) were recorded. Blood pressure was measured at the radial artery or femoral artery, and samples for ABGA were obtained through this artery. After mainstream capnography (CAPNOSTAT M2501A®, Philips, Boblingen, Germany) was available, differences between PaCO2 and ETCO2 were calculated, and the ventilator setting was adjusted to maintain the calculated PaCO2 within the normal range prior to the apnea test. Blood pressure was controlled with dopamine, norepinephrine, and vasopressin, and the target systolic blood pressure was more than 100 mmHg.
Neurologic examination for the declaration of brain death was performed twice, and the apnea test was performed at the end of each examination. In accordance with the apnea test protocol, subjects were preoxygenated with 100% oxygen for 10 minutes prior to the test. The beginning of the test was defined as the time of disconnecting subjects from the ventilator, and 100% oxygen was delivered to the endotracheal tube of subjects via the T-piece. The physician looked closely for respiratory movement, and ETCO2 was monitored if capnography was used.
ABGA was performed 1-3 minutes after the beginning of the apnea test. If PaCO2 was not greater than 50 mmHg, additional ABGA was performed at intervals of 1-3 minutes. When PaCO2 reached the target value and there was no spontaneous respiratory movement, the apnea test was ended and declared positive. If serious complications, such as severe hypoxemia (pulse oximetry saturation [SpO2] < 90%), hypotension (systolic blood pressure [SBP] < 90 mmHg), or critical cardiac arrhythmia, occurred before or during the apnea test, the test was terminated. If PaCO2 did not reach the target level up to that time, a test for cerebral blood flow (transcranial Doppler ultrasonography or cerebral angiography) was used to replace the apnea test.
The subjects were divided into two groups: the N-group, in which capnography was not used, and the C-group, in which capnography was used to monitor ETCO2. The time to target PaCO2 (min) was defined as the period from the beginning of apnea test to the first ABGA sampling of the target PaCO2. The total apnea test period (min) was defined as the period from the beginning of the test to the actual end of the test after confirming the target PaCO2. The attending ICU nurse recorded the arterial blood pressure, pulse rate, and pulse oximetry saturation at the beginning and end of the apnea test in all subjects.
SPSS for Windows (version 18.0, SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Categorical variables were analyzed using the chi-square test or Fisher's exact test. Continuous variables were tested for normality using the Kolmogorov-Smirnov test. Then, the variables showing normality were analyzed using the Student's t-test and expressed as the mean ± standard deviation. The variables not showing normality were analyzed using the Mann-Whitney test and expressed as the median (25th percentile-75th percentile). All P values were calculated using a 2-tailed test, and a P value of less than 0.05 was considered statistically significant.
Results
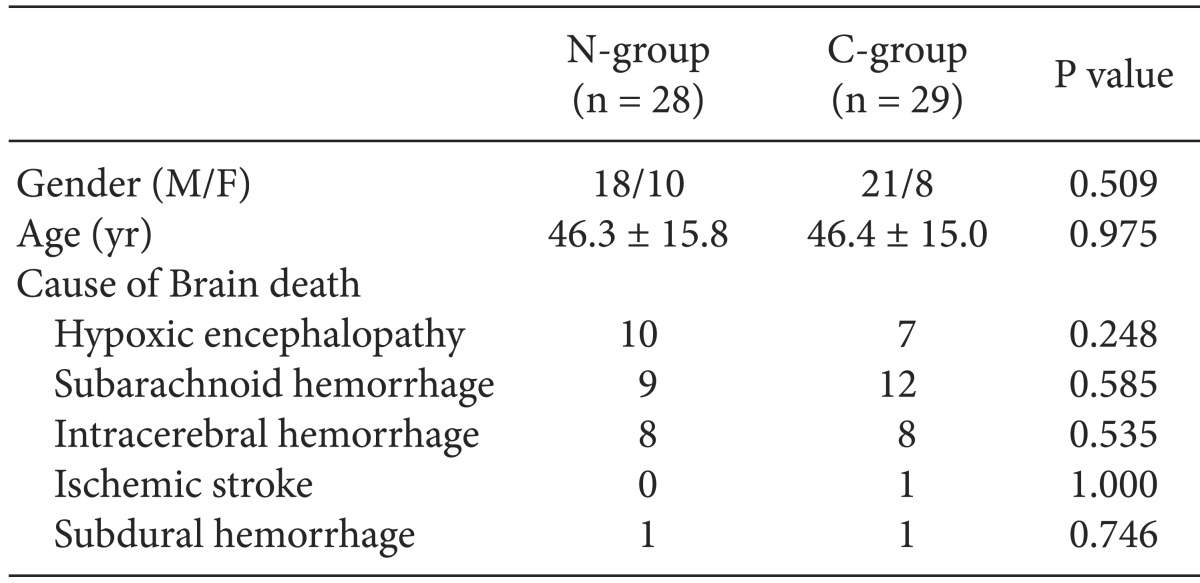
The N-group consisted of 31 patients, and the C-group had 30 patients. Three (9.7%) patients in the N-group were excluded due to uncorrected hypotension (SBP < 90 mmHg) before the apnea test, and one patient (3.3%) in the C-group was excluded due to hypoxemia (SpO2 < 90%) before the apnea test. Therefore, the final sample comprised 28 patients in the N-group and 29 patients in the C-group. No differences were observed between the two groups in gender, age, and cause of brain death (Table 1).
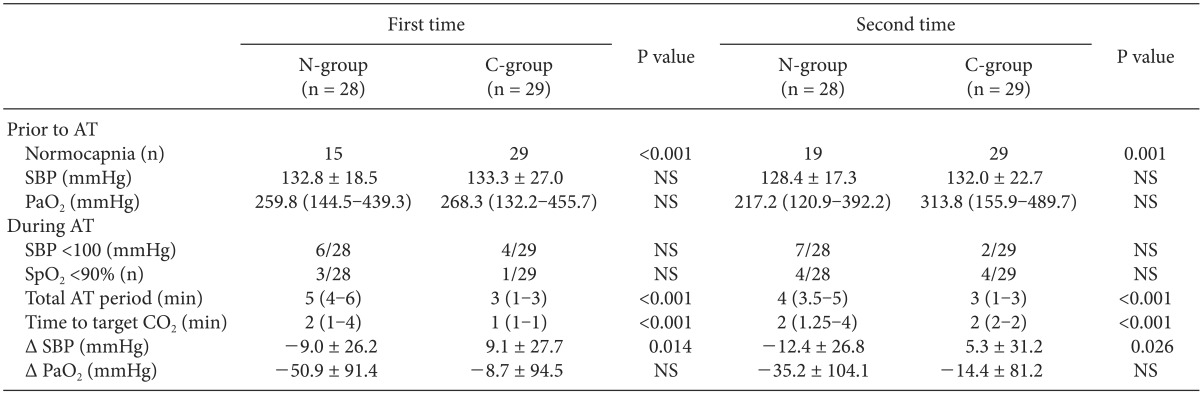
Because the apnea test was performed twice in each patient, ABGA results and hemodynamic variables are arranged separately for each test (Table 2). The ratio of normal PaCO2 before the test (%), total test time (min), and time to target PaCO2 (min) were significantly different between the two groups. The SBP of the N-group decreased during the test (1st AT: -9.0 ± 26.2 mmHg, 2nd AT: -12.4 ± 26.8 mmHg), while the SBP of the C-group increased during the test (1st AT: 9.1 ± 27.7 mmHg, 2nd AT: 5.3 ± 31.2 mmHg). The SBP changes during the apnea test were significantly different between the two groups (1st AT P value: 0.014, 2nd AT P value: 0.026). During the test, there were fewer subjects with SBP less than 100 mmHg in the C-group than in the N-group, although there were no significant differences. During the test, there were fewer subjects with SpO2 less than 90% in the C-group than in the N-group, and PaO2 was less decreased in the C-group than in the N-group, but there was no statistical significance.
Discussion
At present, about 10 countries approve a legal definition of brain death; these include the U.S., Canada, France, Japan, and Taiwan. Furthermore, more than 30 countries allow organ transplantation from brain dead patients. In 1968, Harvard Medical School set forth criteria for diagnosis brain death, and since then, various countries have furnished their own guidelines for the diagnosis of brain death. Prerequisites (i.e., types of confirmatory and mandatory tests for the declaration of brain death) differ slightly, but the differences are minimal from a medical standpoint. In 1993, the Korean Medical Association established criteria for organ transplantation, and in February 1999, The Act on Organ Transplantation was enacted [12]. In fact, however, organ transplantation had been performed since 1992, before the legislation passed; in spite of the lack of legal recognition of brain death, medical professionals approved and performed organ transplantation. The number of organ transplantations from brain dead patients has increased steadily, and during 2012, there were 409 brain dead donors [13].
The legislated rules for the diagnosis of brain death proposed by the Korea Medical Association list seven categories. In these rules, the apnea test for evaluating the recovery of respiratory drive is described as follows.
"After preoxygenation with 100% oxygen or 95% oxygen and 5% carbon dioxide for 10 minutes, disconnect the patient from the ventilator and deliver 6 L/min of 100% oxygen through the endotracheal tube. Look closely for 10 minutes, and if respiratory movements are absent in spite of PaO2 > 50 mmHg, it is determined that spontaneous respiration cannot be revived. If the apnea test was not performed completely or was stopped midway, accessorial tests such as a cerebral blood flow study should be performed."
Although the apnea test is a confirmatory test in various countries, the target PaCO2 level during the test differs between countries. While some countries, such as the U.S., Canada, and Germany, set the target PaCO2 level at 60 mmHg, other countries such as Korea, the U.K., Switzerland, and Portugal set 50 mmHg as the target PaCO2 level. These differences are not meaningful from a medical standpoint, given that the optimal level for sufficiently stimulating the respiratory center is unknown [14]. However, based on the difference in target PaCO2, we expect to be able to reduce the duration of the apnea test more effectively in Korea than in other countries.
The apnea test can cause serious complications such as severe respiratory acidosis, hypotension, and hypoxemia. As these can worsen the donor's condition and increase the failure rate of organ procurement, we should optimize the donor's condition and reduce the period of apnea testing. However, because the rules enacted by law do not specify patient condition in detail, it is unfeasible to perform the apnea test based only on these rules. Each hospital therefore sets detailed guidelines for the declaration of brain death. In many hospitals, such guidelines are based on the AAN guidelines [15]. Our hospital also set guidelines based on those of the AAN (Table 3). Our guidelines recommend maintaining a normal PaCO2 level (35-45 mmHg) before the apnea test and preventing severe hypotension and hypoxemia. If this is difficult, the test should be terminated and replaced with an alternative test. However, to maintain PaCO2 within a normal range, repeated ABGA samplings are needed, which causes increased labor and cost. We devised capnography to replace ABGA samplings to check PaCO2 levels. Up to now, we could not find any study on the usefulness of capnography in brain dead donors. Thus, we planned a study on the usefulness of capnography in apnea testing.
The human body releases carbon dioxide (CO2) accumulated in the process of metabolism by ventilation through the airway. Therefore, PaCO2 can be predicted by the measurement of ETCO2 using capnography. In normal lungs, PaCO2 is higher than ETCO2 by 2 to 5 mmHg. This is why dead space in lungs dilutes the CO2 concentration in the actually ventilated amount of CO2. Dead space can be divided into anatomic dead space and physiologic dead space. Anatomic dead space is not part of the ventilating airways, such as terminal bronchioles and alveoli, but rather a part of the conducting airways. Dead space can also exist in the distal part of anatomic dead space in the presence of ventilation-perfusion mismatch; this is called physiologic dead space. Because anatomic dead space is fixed whereas physiologic dead space changes with variation in ventilation and perfusion, if physiologic dead space increases, the gradient of PaCO2 and ETCO2 increases [16,17,18]. In research involving patients on long-term mechanical ventilation, such as brain dead donors, the gradients increased in those patients more than in patients with normal lung condition [19,20,21]. In studies on brain dead donors, Vivien et al. [18] reported 9 ± 4 mmHg and Sharpe et al. [22] reported 7 ± 4 mmHg. From these results, it can be seen that brain dead donors have a larger gradient of PaCO2 and ETCO2 than do patients with normal lung condition. In the present study, the gradient of PaCO2 and ETCO2 is also large: 7 ± 5 mmHg the first time and 6 ± 6 mmHg the second time. Such results are explained by the fact that long-term mechanical ventilation increases atelectasis and ventilation-perfusion mismatch [23].
In the C-group, we calculated the gradient of PaCO2 and ETCO2 whenever ABGA was sampled, while continuously monitoring ETCO2 in each patient before the apnea test. Moreover, by taking the gradient into consideration, we adjusted mechanical ventilation to maintain PaCO2 within the normal range. Thus, we were able to monitor PaCO2 continuously in the C-group in contrast to the N-group, in which PaCO2 was monitored intermittently by ABGA samplings. As a result, the ratio of normal PaCO2 was 100% in the C-group both times, but in the N-group, the ratio of normal PaCO2 was 15/28 (53.6%) the first time and 19/28 (67.9%) the second time; there was a significant difference between the two groups.
During the apnea test, because CO2 in the blood cannot be released to alveoli, respiratory acidosis and hypercarbia begin to progress. Accumulated CO2 and acidosis caused by increased CO2 lead directly to reductions in peripheral arteriolar resistance, cardiac contractile force, and the rate of conduction. In the sympathetic system, however, the opposite action occurs. Aortic and carotid chemoreceptors stimulated by hypercarbia indirectly increase the activation of sympathetic tone, which improves cardiac contractile force and vascular resistance. Therefore, overall hemodynamic changes are impacted by a combination of direct and indirect effects. Because excessive acidosis reduces the sympathetic response of the human body, so the effect of cardiovascular depression by acidosis and hypercarbia exceed the effect of cardiovascular stimulation by sympathetic response [24,25]. Therefore, in delayed apnea status during apnea testing, hemodynamic instability progresses, and critical complications such as severe hypotension, arrhythmia, and cardiac arrest are likely to occur [3,4,5]. For that reason, it is necessary to minimize the apnea test period as well as maintain a normal range of PaCO2. The rate at which PaCO2 increases in the apnea test is non-linear; if PaCO2 is in the range of about 40-60 mmHg, the rate of increase is 4-6 mmHg/min, and if PaCO2 is in a higher range, the rate of increase is 2 mmHg/min. In addition, recent studies show variation in the rate of PaCO2 increase. Vivien et al. [18] reported 2.8 ± 0.9 mmHg/min, Edward et al. [26] reported 3.7 ± 2.3 mmHg/min, and Bruce [27] reported 1.7 mmHg/min. This variation is caused by passive CO2 washout, atelectasis, cardiac-induced ventilations, and other unknown factors [26]. Thus, even though we know the value of PaCO2 before the apnea test, it is difficult to predict the time to target PaCO2 (50 mmHg) after the start of the test. We assume, however, that maintaining a normal PaCO2 level with ETCO2 monitoring can reduce the duration of the apnea test compared to no monitoring of ETCO2. As we expected, in the C-group, the time to target PaCO2 and total apnea test period were reduced significantly compared to the N-group (Table 2).
We also expected to reduce hemodynamic changes and hypoxemia by reducing the total test time in the C-group. To confirm the reduction of hemodynamic changes, we recorded SBPs at the beginning and end of the test and checked the differences. Differences in SBP during the test (SBP at the end of AT - SBP at the beginning of AT) were found in the N-group (1st AT: -9.0 ± 26.2 mmHg, 2nd AT: -12.4 ± 26.8 mmHg) and in the C-group (1st AT: 9.1 ± 27.7 mmHg, 2nd AT: 5.3 ± 31.2 mmHg). In the C-group, SBP increased at the end of the test compared to the beginning both times the test was administered. Although the usage of inotropics differed for each patient, the protocol regarding their use was the same-to maintain SBP at more than 100 mmHg. Thus, we think the reason that SBP increased only in the C-group is that the reduction in the total apnea test period and the prevention of excessive acidosis contributed to hemodynamic stability. As mentioned before, it is known that in the initial stage of the apnea test, a temporary increase in BP can appear due to sympathetic stimulation, and a decrease in BP appears as acidosis progresses. To confirm a temporary increase in BP in the initial stage of the apnea test, it would be necessary to look for a change in BP as time progressed in both groups, but we could not find the records of real-time BP during apnea testing. Thus, we instead compared the incidence of SBP under 100 mmHg with both groups according to whether PaCO2 was within the normal range. The incidence of SBP under 100 mmHg during the first test (normal PaCO2 group 8/44 [18%], abnormal PaCO2 group 2/13 [15%]) and second test (normal PaCO2 group 6/48 [12%], abnormal PaCO2 group 3/9 [33%]) was identified. In short, we found no difference in the incidence of SBP under 100 mmHg between the normal and abnormal PaCO2 groups. The ratio of SpO2 under 90% during the apnea test for the first test (N-group 3/28 [10.7%], C-group 1/29 [3.4%]) and second test (N-group 4/28 [14.3%], C-group 4/29 [13.8%]) was also determined. Although the N-group had a higher incidence than the C-group, there was no significant difference.
The ultimate goal of this study was to minimize damage to organs planned for transplantation and thus contribute to successful organ transplantation. We thus examined whether available organs and final donated organs were different between the N-group and C-group. The discrepancy between available organs and final donated organs was 8/28 (29%) in the N-group and 12/29 (41%) in the C-group, not a significant difference (P value 0.31). However, we could not evaluate the relationship between the hemodynamic stability of donors and the success rate of organ transplantation because of a number of variables we could not identify, including problems related to organ extraction from donors, status change of recipients, donor-recipient mismatch, and problems related to organ transportation. Such issues are left to further studies.
There are some limitations to the present study. First, because this is a small group study that examined only 57 patients, it is possible that a larger sample might yield statistically significant results that did not achieve significance in this study. Second, because we had trouble collecting data due to the nature of retrospective research, we could not confirm some variables we hoped to identify, such as hemodynamic changes during the apnea test and the rate of successful transplantation. Third, as the examinations were performed by various physicians, it is possible that different methods were used to diagnose brain death. The results showed differences in the interval from the beginning of the test to first blood sampling, and, if first PaCO2 did not reach target value, the interval from first blood sampling to second blood sampling. Thus, comparing the individual durations of the apnea tests is difficult. However, it remains the case that there were statistically significant differences between the groups. Fourth, because this investigation covered more than 3 years, differences in the skills of the examiner could occur with the passage of time.
In summary, we maintained PaCO2 within a normal range when monitoring ETCO2 before apnea testing. In addition, because maintaining PaCO2 within a normal range reduced the time to reach target PaCO2 from the beginning of the test, the total apnea test period was reduced. Based on the reduction of the test period, we confirmed that SBP increased more at the end of the test than the beginning in the group in which capnography was applied. Thus, when applying capnography in brain dead patients, we confirmed that the duration of the apnea test is reduced and hemodynamic stability is increased. In conclusion, ETCO2 monitoring with capnography is helpful when performing the apnea test.