|
 |
|
|
Abstract
Endovascular repair with covered stents has been widely used to treat subclavian and axillary artery injuries and has produced promising early results. The possibility of a thromboembolism occurring in cerebral arteries during an endovascular procedure should be a cause for concern. In the case of endovascular management of arterial traumas, a prompt and sufficient period for check-up of the patient's neurological signs is needed, even if it requires postponing elective intervention for the patient's safety. We report a rare case of liver transplantation immediately after endovascular repair of an iatrogenic subclavian arterial injury to describe the risk of continuing planned surgery without neurologic assessment.
Traumatic injuries of subclavian arteries are associated with high morbidity and mortality [1]. Although surgical repair of these vessels is recognized as the first-line treatment, most clinicians hesitates surgical approach due to difficulty in exposure of the vessels and possible complication such as brachial plexus injuries, pulmonary contusions, and bony fractures [2].
Recently, an endovascular repair with covered stents has been used widely to treat subclavian and axillary artery injuries and has produced promising early results despite of uncertainties in patient selection and optimal management algorithms [3].
This report describes a patient who underwent consecutive liver transplantation surgery immediate after endovascular repair of iatrogenic subclavian arterial injury that occurred during anesthetic induction without neurological assessment, revealed subacute multiple air embolic infarction 8 days after surgery.
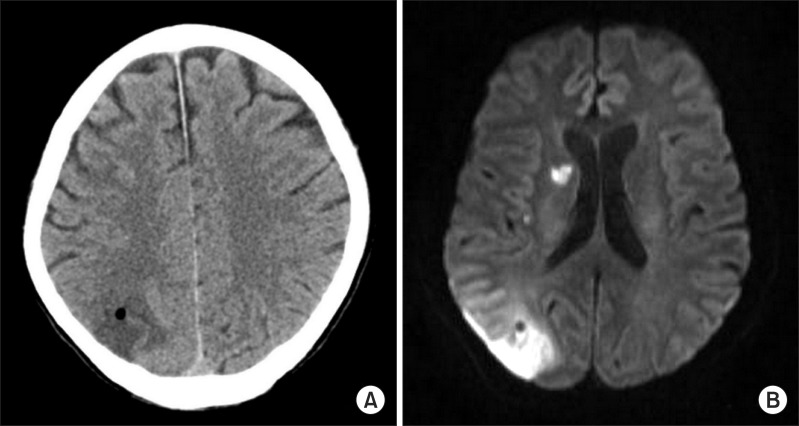
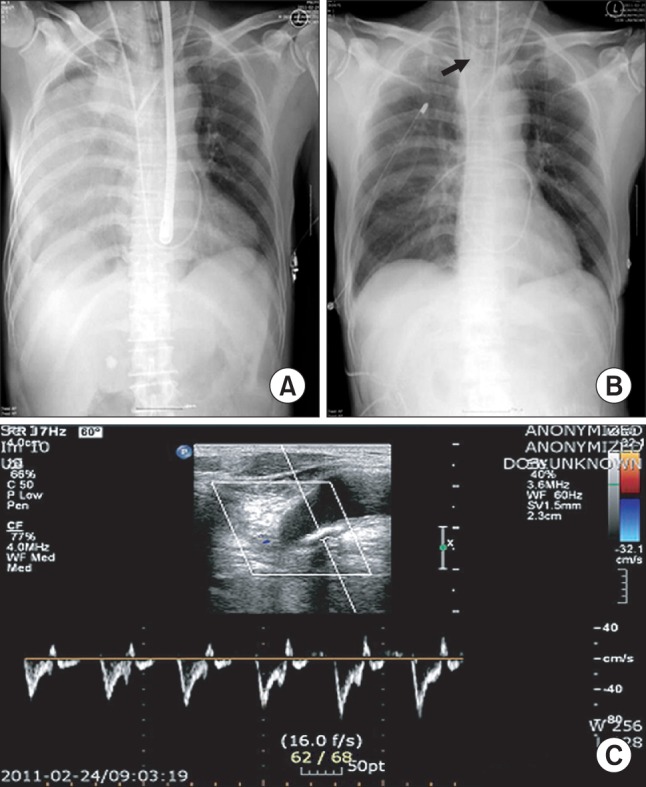
A 60-year-old man who was diagnosed with liver cirrhosis and hepatocellular carcinoma with splenomegaly due to hepatitis B virus (Child-Pugh grade A, MELD score 14) presented for living donor liver transplantation. Blood tests before surgery indicated that the laboratory values of platelet counts (64,000 /mm3), while the other laboratory findings were within normal range (international normalized ratio: 1.12, activated partial thromboplastin time: 39.9 sec). Electrocardiography, transthoracic echocardiography and chest X-ray tests appeared normal. Anesthesia was induced with midazolam (10 mg) and rocuronium (0.8 mg/kg) and maintained with sevoflurane in air and oxygen. Routine vascular access including cannulation at the right internal jugular vein, subclavian vein, radial artery, femoral artery and femoral vein for liver transplantation were attempted Cannulation at the right internal jugular vein was attempted by inserting a Swan-Ganz catheter into the pulmonary artery under ultrasonographic guidance with an anterior approach using the Seldinger technique. The femoral vein cannulation for detection of incidental compression of inferior vena cava was also performed without issues. The cannulation of the radial and femoral artery was performed using 20 G intravenous catheter without any complications. A 12 Fr large bore catheter (Arrow-HowesŌäó large-bore multi-lumen central venous catheter, Arrow international, Reading, PA, USA) into right subclavian vein was performed, an 18 G puncture needle was inserted by the infraclavicular approach, backflow of venous blood via the puncture needle was confirmed and a guidewire was threaded through a needle without difficulty. Some resistance was felt on advance of a dilator device during dilatation of the puncture hole; therefore, the guide wire and dilator were removed, and we confirmed a distorted guide wire. Under ultrasonographic guidance, a 12 Fr large bore catheter was inserted into the left internal jugular vein without issue. As a routine method, a rapid infusion system and cardiovascular drug infusion line were connected through the left internal jugular vein. The patient showed abrupt changes in vital signs with a heart rate of 130 beats/min, blood pressure of 50/30 mmHg and end tidal carbon dioxide at 18 mmHg. Packed RBC 4 uints, fresh frozen plasma 4 units, 1,000 ml of crystalloids and 200 ml of 20% albumin were infused through rapid infusion system. Inotropes and vasopressors (dopamine 8 ┬Ąg/kg/min, dobutamine 8 ┬Ąg/kg/min, norepinephrine 0.1 ┬Ąg/kg/min) infusion started. And then the vital signs were recovered. Arterial blood gas analysis was done and revealed pH 7.403, PaO2 of 150 mmHg, and PaCO2 of 39.2 mmHg with FiO2 0.6 and hemoglobin at 12.2 g/dl. Although these results did not differ from previous arterial blood gas analyses, we decided to perform transesophageal echocardiography to detect possible problems such as cardiac dysfunction, pneumothorax, or hemothorax. An anechoic feature was seen in the right lung area suggesting a hemothorax, and then we confirmed a hemothorax by portable chest radiography (Fig. 1A). Using ultrasonography on supraclavicular area, we confirmed penetrated window and leaks of blood from right subclavian artery and right common carotid artery bifurcation of brachiocephalic trunk to right pleural cavity due to right subclavian artery injury (Fig. 1C). The vital signs were stable under direct compression of the injured site. Donor hepatectomy was initiated for transplantation; therefore, we could not delay surgery for further evaluation. Radiologic intervention or surgical repair was necessary for performing this surgery because of the use of anticoagulants after implantation of the donated liver. The radiologist and vascular surgeon decided to insert a stent graft by retrograde transbrachial approach in the operation room. An endovascular stent graft (JOSTENT, peripheral stent-graft 6-12 mm ├Ś 28 mm bare type, Abbott Vascular Ltd, Rangendingen, Germany) was implanted to cover the arterial laceration. A closed thoracostomy tube was placed for management of the hemothorax (Fig. 1B). Shortly after the thoracotomy, 2,000 ml of fresh blood was directly drained off and drainage was slowly deceased. At the same time the live donor, the patient's son, was undergoing right hepatectomy. Therefore, the surgeon decided to conduct liver transplantation as planned. During the operation, the vital signs were stable and the amount of drainage via the chest tube was 600 ml. The operation ended without problems and the patient was sent to an intensive care unit. We confirmed appropriate stent position, which did not compromise the right vertebral artery and carotid flow, by angiography. The patient awoke next day and was transferred to the general ward after 4 days stay in the intensive care unit. At 8th postoperative day, patient has presented with neurologic signs such as mental change, left homonymous hemianopsia and left upper arm weakness. Brain computed tomography and Magnetic resonance imaging was demonstrated multiple embolic infarction in right hemisphere and cerebellum due to air embolism. The radiologist confirmed that it was a subacute infarction and the air embolism could have occurred during the endovascular stent graft procedure because the infarction site was the occipital area, which originates from the right vertebral artery (Fig. 2). Although the patient spontaneously recovered mental function and left upper arm weakness on the next day, he complained of left hemianopsia and was discharged 6 weeks after surgery.
Up to date, endovascular repair has been primarily used to treat certain type of artery injuries, however the indications and management guidelines for endovascular repair are still lacking. In addition, although many reports have suggested strokes as a possible complications related to endovascular repair, there is no document dealt with practical problem such as 'When occurs stroke after endovascular repair?', 'How long do we have to observe patient's neurologic sign after endovascular repair?'. This case showed subacute embolic infarction could occur even 8 days after surgery.
Arterial injury occurs 0.1-0.8% of population during central vein catheterization [1]. It is associated with life-threatening complications including arteriovenous fistulas, pseudoaneurysms, hemothorax, strokes, and potential airway obstruction. Through our case, we assumed arteriovenous fistula lesion was formed by inadvertent insertion of dilator. Arterial injury can be managed by external compression, endovascular stent graft insertion, or by surgical exploration and direct arterial repair. External compression with subsequent observation has been commonly conducted in the clinical setting. However, this technique is associated with significantly higher morbidity (stroke, suddenly expanding hematoma causing airway compression, false aneurysm) than a surgical or endovascular approach, with a relative risk of 17.86 favoring surgical or endovascular repair and a number needed to treat of 1.5 [4]. Directed surgical repair of a subclavian artery injury may be complicated due to difficulties exposing the surgical field and the requirement for partial rib resection and thoracotomy. In contrast to these techniques, endovascular management of a subclavian artery is a safe and minimally invasive technique than surgical repair, because a median sternotomy or thoracotomy, less blood loss, and fluid shifts is avoided [5]. DuBose et al. [3] reported that endovascular treatment of traumatic subclavian and axillary artery injuries continue to evolve with promising early results; successful initial endovascular device placement occurred in 96.9% of the treated patients with longer-term patency in 84.4% over a follow-up period varying from 0 months at the time of hospital discharge to 70 months. Despite growing enthusiasm for endovascular repair of axillary and subclavian vessel injuries, realistic clinical presentation and anatomic locations restrict the broad application of this technique. Danetz et al. [6] reported that less than 50% of all injuries could be addressed with an endovascular approach, and an algorithm for selection of the patient is needed. Some investigators have proposed an algorithm for cervico-thoracic or subclavian artery injuries and recommended stable vital signs and accessibility by imaging the injury site [4,7]. In addition, total vessel transaction and refractory hypotension were regarded as contraindications for endovascular repair [3].
Several complications can occur following an endovascular approach. Access site hematoma or pseudoaneurysms are the most commonly reported complications [8,9]. Moreover, endovascular management can increase the risk of occlusion of the origins of the internal mammary and vertebral arteries, acute thrombosis, compromising the common carotid artery flow, delayed intimal fibrosis or stenosis, and stent fracture due to compression between the clavicle and first rib. The potential for thromboembolism in cerebral arteries during an endovascular procedure, especially in the right subclavian artery, should be a cause of concern as it leads to neurologic problems. The occurrence of air embolism may be also hazardous. Catheter flushing with saline, angiography, and stent deployment could be the source of air embolism during endovascular repair [10].
Cerebral thromboembolism could also induced by liver transplantation surgery due to peri-operative detachment of arterial emboli from carotid or intracranial arteries or paradoxical emboli of thrombotic material from deep leg [11]. Thus, we could differentiate our case from those occurred after liver transplantation by prominent air embolic signal (Fig. 2).
Guilbertand et al. [4] recommended that after arterial repair, prompt neurological evaluation should be performed, even if it requires postponing elective intervention. In addition, although many studies about cerebral embolism after carotid endarterectomy have been reported [12], no reviewed studies about the incidence of cerebral embolism after a subclavian arterial stent have been reported in PubMed. Strokes have been regarded as an early complication, occurring within 24 h after an intervention; 24 h serial clinical follow-up to exclude neurological complication have been recommended. However, in our case, it was very difficult to stop liver transplantation for neurologic evaluation, because hepatectomy in the donor patient had begun. We decided to conduct endovascular management in the operation room and continued liver transplantation as scheduled. During the liver transplantation operation, we performed neurologic monitoring such as bispectral index scale and cerebral oximetry, but we did not conduct transcranial Doppler (TCD) monitoring for detectiona of a thromboembolism. Although embolus detection and differentiation is very difficult when there are bursts of gaseous or solid emboli, there still is some debate on the prediction of a cerebral embolism by TCD [13]. The criteria for detection and differentiation of cerebral embolism using multifrequency TCD as follows [14]: Doppler signal enhancement, embolus-blood-ratio (EBR), is > 28 dB/ms (for example, a Doppler power increase > 7 dB, which lasts > 4 ms) simultaneously in 2.0 and 2.5 MHz channels. In our case, on site TCD would have been helpful to detect a cerebral air embolism and neurologic assessment during liver transplantation, and led to earlier diagnosis of the cerebral embolic infarction.
Based on this case, we suggest that in the case of endovascular management of arterial trauma, selection of the patient and amenability for conducting endovascular management and a prompt and sufficient check-up period of the patient's neurological signs for the patient's safety are needed, even if it requires postponing elective intervention. In addition, if continuation of surgery is unavoidable after endovascular repair, reliable neurologic monitoring on site such as TCD is needed.
References
1. Demetriades D, Asensio JA. Subclavian and axillary vascular injuries. Surg Clin North Am 2001; 81: 1357-1373. PMID: 11766180.


2. Althaus SJ, Keskey TS, Harker CP, Caldwell DM. Percutaneous placement of self-expanding stent for acute traumatic arterial injury. J Trauma 1996; 41: 145-148. PMID: 8676409.


3. DuBose JJ, Rajani R, Gilani R, Arthurs ZA, Morrison JJ, Clouse WD, et al. Endovascular management of axillo-subclavian arterial injury: a review of published experience. Injury 2012; 43: 1785-1792. PMID: 22921384.


4. Guilbert MC, Elkouri S, Bracco D, Corriveau MM, Beaudoin N, Dubois MJ, et al. Arterial trauma during central venous catheter insertion: Case series, review and proposed algorithm. J Vasc Surg 2008; 48: 918-925. PMID: 18703308.


5. Castelli P, Caronno R, Piffaretti G, Tozzi M, Lagan├Ā D, Carrafiello G, et al. Endovascular repair of traumatic injuries of the subclavian and axillary arteries. Injury 2005; 36: 778-782. PMID: 15910833.


6. Danetz JS, Cassano AD, Stoner MC, Ivatury RR, Levy MM. Feasibility of endovascular repair in penetrating axillosubclavian injuries: a retrospective review. J Vasc Surg 2005; 41: 246-254. PMID: 15768006.


7. Carrick MM, Morrison CA, Pham HQ, Norman MA, Marvin B, Lee J, et al. Modern management of traumatic subclavian artery injuries: a single institution\'s experience in the evolution of endovascular repair. Am J Surg 2010; 199: 28-34. PMID: 19520356.


8. Hilfiker PR, Razavi MK, Kee ST, Sze DY, Semba CP, Dake MD. Stent-graft therapy for subclavian artery aneurysms and fistulas: single-center mid-term results. J Vasc Interv Radiol 2000; 11: 578-584. PMID: 10834488.


9. Schoder M, Cejna M, H├Člzenbein T, Bischof G, Lomoschitz F, Funovics M, et al. Elective and emergent endovascular treatment of subclavian artery aneurysms and injuries. J Endovasc Ther 2003; 10: 58-65. PMID: 12751932.



10. Bendszus M, Koltzenburg M, Burger R, Warmuth-Metz M, Hofmann E, Solymosi L. Silent embolism in diagnostic cerebral angiography and neurointerventional procedures: a prospective study. Lancet 1999; 354: 1594-1597. PMID: 10560674.


11. Amodio P, Biancardi A, Montagnese S, Angeli P, Iannizzi P, Cillo U, et al. Neurological complications after orthotopic liver transplantation. Dig Liver Dis 2007; 39: 740-747. PMID: 17611177.


12. Halliday A, Mansfield A, Marro J, Peto C, Peto R, Potter J, et al. Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomized controlled trial. Lancet 2004; 363: 1491-1502. PMID: 15135594.


13. Skjelland M, Krohg-S├Ėrensen K, Tenn├Ėe B, Bakke SJ, Brucher R, Russell D. Cerebral microemboli and brain injury during carotid artery endarterectomy and stenting. Stroke 2009; 40: 230-234. PMID: 18927460.


14. Russell D, Brucher R. Online automatic discrimination between solid and gaseous cerebral microemboli with the first multifrequency transcranial Doppler. Stroke 2002; 33: 1975-1980. PMID: 12154248.


Fig.┬Ā1
Chest radiograph (A) showed hemothorax in the right thoracic cavity and chest radiograph (B) showed successfully deployed stent graft (black arrow) and placed thoracotomy tube. (C) showed Pulsed-wave Doppler image, obtained by ultrasonography on supraclavicular area, showed detached arterial wall and leaks of blood from subclavian artery to right pleural cavity.
- TOOLS