|
 |
|
|
Abstract
We experienced a living donor liver transplantation for a 26-month-old girl with complement factor H deficiency. Complement factor H is a plasma protein that regulates the activity of the complement pathway. Complement overactivity induced by complement factor H deficiency is associated with atypical hemolytic uremic syndrome. Liver transplantation can be the proper treatment for this condition. During the liver transplantation of these patients, prevention of the complement overactivation is necessary. Minimizing complement activation, through the use of modalities such as plasma exchange before the surgery and transfusion of fresh frozen plasma throughout the entire perioperative period, may be the key for successful liver transplantation in these patients.
The hemolytic uremic syndrome (HUS) is characterized by microangiopathic hemolytic anemia, thrombocytopenia, and renal impairment. HUS is classified into typical (diarrheal, ~90%) and atypical (non-diarrheal) [1]. Complement factor H (CFH) is a plasma protein synthesized mainly by the hepatocytes [2]. Its central role is down-regulation of activation of the alternative complement pathway [2]. Mutations in the gene encoding CFH are related to disorders of complement regulation [1]. The overactive complement system can create endotheliopathy in the kidneys. CFH deficiency (CFHD) is one of the causes of atypical HUS [1]. CFHD-HUS is a rare disease, but it tends to recur and carries a poor prognosis; the majority of patients will progress to end-stage renal disease (ESRD) or death [3,4]. Liver transplantation (LT) can correct the complement overactivity and prevent recurrence of disease in these patients [4,5,6,7,8,9] because CFH is predominantly produced by the hepatocytes. Despite this theoretical background, however, there have been several case reports of problematic outcomes after trials of LT for treatment of CFHD-HUS [4,9,10]. Histologic assessment suggest that the overactive complement system may have been the cause of the fatal outcomes in these cases [10]. Therefore, minimizing the complement overactivity that is induced by CFHD during surgery may be important for successful LT of the CFHD-HUS patient [5,6,7,8]. We present a successful case report of a patient with CFHD-HUS who underwent living donor LT (LDLT).
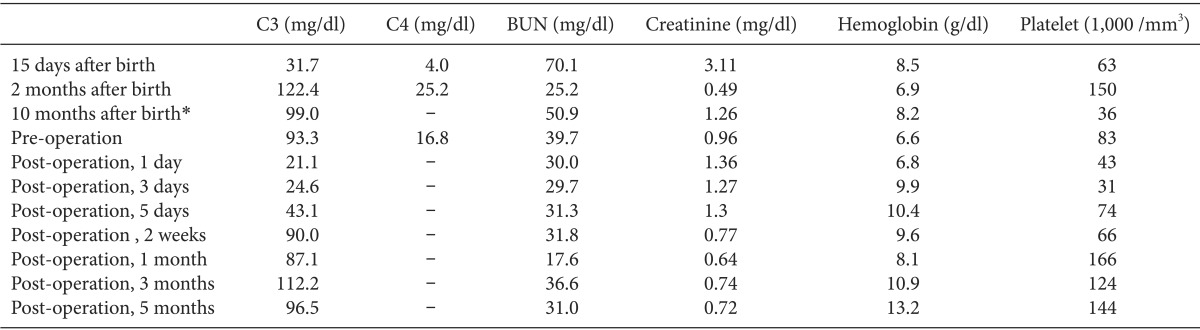
Our patient was born by cesarean section at a gestational age of 38 weeks. Her perinatal history was uneventful. She developed jaundice, persistent thrombocytopenia, and elevated serum creatinine without any signs of infection 3 days after birth. The peripheral blood smear showed schistocytes. Her C3 and C4 concentrations (Table 1) were decreased. The plasma concentrations of CFH proteins analyzed by Western blot (Fig. 1) were 23% of the normal value. At the age of 3 months, the patient was diagnosed with CFHD-HUS by confirmation of a heterozygous mutation in CFH gene sequencing. Her parents showed normal gene sequencing.
After her diagnosis, the patient was treated with fresh frozen plasma (FFP) infusions (10-20 ml/kg) through a central line twice weekly, depending on the disease activity. With that treatment, her renal function and platelet count were well maintained. Her C3 concentration was kept slightly low, with normal levels of C4. However, whenever a central line-related or viral infection occurred, she presented with hematuria, thrombocytopenia, and acute renal failure. After the age of 8 months, the patient was treated with plasma exchange (PE) whenever HUS recurred.
We intended to perform a LDLT before the patient's renal function declined. Although her parents did not show any abnormalities in gene sequencing, neither of them could be considered suitable as potential liver donors because of the possibility that they are carriers of mutations. In addition, the patient would have to undergo prophylactic PE prior to the LT operation. However, if deceased donor LT were to be performed, she would not have sufficient time to undergo PE. Therefore, we had difficulty in identifying a appropriate donor. Fortunately, a volunteer donor appeared and we were able to attempt a LDLT. Because the patient had had a central venous catheter for a long time and had suffered from catheter-related infection and thrombosis several times, we anticipated difficulty in accessing the central venous line. Preoperative ultrasonographic examination revealed occlusions of both internal jugular veins. A Hickman 10.0 French triple lumen catheter had been placed in the left subclavian vein under general anesthesia about 10 days before the operation. One day before the operation, we placed an additional 5.5 French triple lumen catheter in the right femoral vein under sedation using 0.6 mg of midazolam in the ward.
At age 26 months, the patient underwent a LDLT. PE was performed 6 hours before the operation. Her weight was 10.9 kg, hemoglobin 9.0 g/dl, platelet count 96,000 /mm3, and prothrombin time expressed with international normalized ratio (INR) 0.98. Blood urea nitrogen was 39.7 mg/dl, serum creatinine 0.96 mg/dl, and albumin 4.6 g/dl (Table 1). The pulse oximeter, noninvasive blood pressure monitor, and 3-lead electrocardiogram were applied and anesthesia was induced with thiopental sodium 50 mg and sevoflurane, and maintained with isoflurane. Vecuronium was used for muscle relaxation. Arterial cannulations were performed in the right radial artery with a 24-gauge catheter and the left femoral artery with a 20-gauge catheter. Central venous pressure (CVP) was monitored with a previously inserted catheter in the right subclavian vein. We checked complete blood count, coagulation profile, and arterial blood gas analysis every 1 or 2 hours during the operation (Table 2). If there was no evidence of a bleeding tendency in the surgical field, transfusion was not given until the laboratory data met the transfusion criteria. In addition, intraoperative salvaged blood was used to avoid homologous transfusion. Furthermore, we arranged the operation time of the donor and the recipient to reduce ischemia/reperfusion injury by shortening warm ischemic time (21 min) and cold ischemic time (69 min). We also checked the serum C3 levels (normal 88-201 mg/dl) during the anhepatic (56.4 mg/dl) and reperfusion (39.2 mg/dl) phases and at the time of completion of the surgery (38.0 mg/dl) to evaluate complement activation. As soon as induction of anesthesia was finished, we started FFP infusion (2 ml/kg/h), and maintained FFP infusion throughout the operation. The patient also received an additional 2 ml/kg FFP at 10 min before reperfusion. After reperfusion, we began a continuous infusion of dalteparin sodium (20 IU/h). She showed stable hemodynamics immediately after reperfusion without any inotropic support, but required dopamine infusion (5 µg/kg/min) 30 min after reperfusion and norepinephrine infusion (0.05-0.1 µg/kg/min) 3 h after reperfusion in order to maintatin mean arterial blood pressure (ABP) greater than 45 mmHg. The total FFP infusion time was about 450 min. The patient was given 1,730 ml of crystalloid, 100 ml of hetastarch (Hextend® injection 6% 500 ml, CJ Corp., Seoul, Korea), 100 ml of 5% albumin, 170 ml of FFP, 1 unit of leukocyte-depleted RBC, and 120 ml of salvaged blood during the operation. We infused FFP under the guidance of monitoring of CVP, ABP, and urine output. We aimed for a CVP value below 10 mmHg. The total urine output was 210 ml and estimated blood loss, expressed as lost red cell mass, was 130 ml. The surgical procedures were uneventful. The left lateral section of the donor liver, weighing 284 g, was used. No circulatory problems were noted during the surgery.
Following the surgery, the patient was transferred to the intensive care unit, and the endotracheal tube was removed on the fourth postoperative day. For 2 weeks after the operation, additional FFP (10 ml/kg/d) was given. Since then, no signs of HUS recurrence have been observed. Her serum C3 level was increasing during the first 2 weeks after the transplantation. About 3 months after the operation, the patient was discharged from the hospital.
Since CFH is a plasma protein, plasmatherapy is first considered for treatment of CFHD-HUS. Plasmatherapy consisting of FFP infusion or PE can be chosen as an empiric therapy for CFHD-HUS [3,11,12], but it cannot prevent progression to ESRD [4,11], which requires kidney transplantation. LT can prevent the recurrence and progression of HUS, because the transplanted hepatocytes facilitates the production and release of CHF [5,6,7,8]. As a result, LT can provide complete resolution of HUS by normalizing the CFH level.
Despite its theoretical appeal, several reports have described fatal outcomes after initial trials of LT in CFHD-HUS [4,9,10]. Remuzzi et al. [4,10] reported two cases of combined liver-kidney transplantation for this disease. One of the two described early failure of the transplanted liver in a CFHD-HUS patient and claimed the importance of avoiding graft hypoperfusion triggered by hemodynamic deterioration during surgery [10]. Based on the authors' histologic findings, this fatal result might be caused by complement-mediated injury to liver vasculature. Regardless of these disappointments, the case furnished evidence that HUS associated with CFHD could be cured by LT. Recently, however, Saland et al. [5,6,8] reported 4 cases of successful liver-kidney transplantation. They provided preoperative PE and intraoperative plasma infusion (10-20 ml/kg). The main difference between successful and unsuccessful cases was maintaining perioperative plasmatherapy. With this modification, providers could meet with good results when performing LT in a CFHD-HUS patient.
In LT, reperfusion injury is well known as the stimulation which activates the complement system [13,14]. Local complement activation within the graft occurs immediately after reperfusion and leads to systemic complement activation. Bellamy et al. [14] suggested that complement activation seemed to occur during the anhepatic and reperfusion phases, and peaked at graft reperfusion. They also demonstrated that the magnitude of activation of the complement system is closely related to the magnitude of hemodynamic changes. To minimize this activation of the complement system during surgery may be an important key to a favorable outcome [5,7]. PE before surgery and continuous plasma infusion until the liver graft is able to synthesize normal CFH may be critical elements in improving outcomes and preventing disease recurrence. The patient in our case also received PE just before surgery and maintained FFP infusion for 2 weeks after the operation, with reference to the several reported cases. In addition to plasmatherapy, what we can do to minimize complement activation is maintain stable hemodynamic status during the operation. Fortunately, our patient did not show a marked decrease in MAP during the first five min after reperfusion, although she did require inotropic infusion 30 min after reperfusion. The MAP was then maintained between 45 and 55 mmHg by the continuous infusion of inotropics (Table 2).
In CFHD patients, HUS can be prevented with a partial correction of serum CFH levels. Plasma transfusion can increase the serum CFH levels to only subnormal values (15-50% of normal value), but this increase is sufficient to prevent clinical relapse of HUS [11,12]. In our case, however, owing to our lack of prior experience, the fact that the disease was rare but serious, and the absence of a proper therapeutic regimen, effective dose determination for perioperative FFP infusion was obscure. The amount of plasma required to control complement activation might depend on the disease activity and the quality of CFH [7]. We presumed that the optimal dose needed to be determined from the dose that induced disease stabilization during the preoperative period. As soon as she was diagnosed with HUS, our patient was treated with FFP infusions of 10-20 ml/kg depending on the platelet count and serum creatinine level, and she showed good response to therapy. As the operation was expected to take about 10 h, we planned to give FFP at a rate of 2 ml/kg/h (total 20 ml/kg). We had known that maximum complement activation occurs during the reperfusion period, so we gave an additional 2 ml/kg FFP for 3 min before reperfusion. Despite our concern about administering a considerable amount of FFP, the CVP remained below 10 mmHg and there was no sign of volume overload during the surgery, so we were able to infuse FFP at the fixed rate of 2 ml/kg/h. Because there was a considerable amount of bleeding during transplantation, the patient might require additional FFP as a replacement of the losses. After the operation, we maintained the FFP infusion (10 ml/kg/d) for 2 weeks. Plasma supplementation needs to be maintained until the grafted liver can produce normal factor. The amount of required plasma may also depend on how long it takes for the transplanted liver to recover its normal synthetic function. In our case, the patient's serum C3 level increased during the first 2 weeks after transplantation.
For indicators of complement activation during LT, we checked serum C3 levels during the anhepatic and reperfusion phases. However, we could not make decisions on the exact amount of FFP required based on the C3 levels during the surgery, because it took time to get the test results for the C3 concentration. Moreover, as the change in C3 during liver transplantation is not known, intraoperative changes in C3 in our case do not show whether the proper amount of FFP was infused. Our patient actually showed markedly decreased serum C3 levels at the anhepatic and reperfusion phases despite our efforts to control the levels. The alternative pathway consumed C3, but not C4. Reduced serum C3 level and a normal C4 in patients with CFHD-HUS suggest selective activation of the alternative pathway [15]. However, at the reperfusion phase in LT, serum concentrations of both C3 and C4 were decreased [14]. This means that not only alternative pathway but also classical pathway activation may have occurred. In addition, C3 levels tend to remain depressed with this disease, irrespective of effective plasmatherapy [11,12]. For this reason, even if the patient received the proper amount of FFP, it could not prevent a decrease in C3 during surgery. By subsequently comparing the C3 change during LT in the patient with normal CFH, the amount of plasma required can be estimated. In theory, in addition to checking C3 levels, measuring plasma CFH by Western blot during surgery may help to evaluate the response for FFP infusion. Measuring CFH during surgery cannot verify response for FFP infusion promptly, but can serve as further reference data for anesthetic management of patients with this disease later.
In addition to plasmatherapy, the authors of successful cases recommended the use of prophylactic anticoagulation (low molecular weight heparin and low-dosage aspirin) because coagulation pathways themselves can activate the complement system [7]. We believed supplementing of functional CFH to be enough to prevent complement-mediated endotheliopathy. Additionally, in all patients undergoing liver transplantation, we have infused dalteparin sodium (low molecular weight heparin) just after the graft reperfusion phase. We did not introduce further anticoagulation therapies because we thought that these would aggravate bleeding tendencies. In place of additional anticoagulation, we employed much higher values of transfusion triggers than those in usual surgical cases to reduce the thromboembolic risk (RBCs were transfused when hemoglobin < 8.0 g/dl, platelets were transfused when platelet count < 30,000 /mm3, FFP was infused when INR > 3.0, and cryoprecipitates were given when fibrinogen < 80 mg/dl).
In conclusion, as CFHD-HUS is very rare, more experience and research are needed to establish the proper therapeutic regimen. Currently, the only, although most important, things we can do in patients with this disease who undergo LT may be providing perioperative plasmatherapy and maintaining hemodynamic stability during surgery. In addition, understanding the status of the patient and the pathophysiology of the disease, and relieving complement activation during surgery are important for successful LT anesthesia in patients with CFHD-HUS.
References
1. Warwicker P, Goodship TH, Donne RL, Pirson Y, Nicholls A, Ward RM, et al. Genetic studies into inherited and sporadic hemolytic uremic syndrome. Kidney Int 1998; 53: 836-844. PMID: 9551389.


2. Estaller C, Schwaeble W, Dierich M, Weiss EH. Human complement factor H: two factor H proteins are derived from alternatively spliced transcripts. Eur J Immunol 1991; 21: 799-802. PMID: 1826264.


3. Zimmerhackl LB, Besbas N, Jungraithmayr T, van de Kar N, Karch H, Karpman D, et al. Epidemiology, clinical presentation, and pathophysiology of atypical and recurrent hemolytic uremic syndrome. Semin Thromb Hemost 2006; 32: 113-120. PMID: 16575686.



4. Remuzzi G, Ruggenenti P, Codazzi D, Noris M, Caprioli J, Locatelli G, et al. Combined kidney and liver transplantation for familial haemolytic uraemic syndrome. Lancet 2002; 359: 1671-1672. PMID: 12020532.


5. Saland JM, Emre SH, Shneider BL, Benchimol C, Ames S, Bromberg JS, et al. Favorable long-term outcome after liver-kidney transplant for recurrent hemolytic uremic syndrome associated with a factor H mutation. Am J Transplant 2006; 6: 1948-1952. PMID: 16889549.


6. Jalanko H, Peltonen S, Koskinen A, Puntila J, Isoniemi H, Holmberg C, et al. Successful liver-kidney transplantation in two children with aHUS caused by a mutation in complement factor H. Am J Transplant 2008; 8: 216-221. PMID: 17973958.


7. Saland JM, Ruggenenti P, Remuzzi G. Liver-kidney transplantation to cure atypical hemolytic uremic syndrome. J Am Soc Nephrol 2009; 20: 940-949. PMID: 19092117.


8. Saland JM, Shneider BL, Bromberg JS, Shi PA, Ward SC, Magid MS, et al. Successful split liver-kidney transplant for factor H associated hemolytic uremic syndrome. Clin J Am Soc Nephrol 2009; 4: 201-206. PMID: 19005013.



9. Cheong HI, Lee BS, Kang HG, Hahn H, Suh KS, Ha IS, et al. Attempted treatment of factor H deficiency by liver transplantation. Pediatr Nephrol 2004; 19: 454-458. PMID: 14986080.


10. Remuzzi G, Ruggenenti P, Colledan M, Gridelli B, Bertani A, Bettinaglio P, et al. Hemolytic uremic syndrome: a fatal outcome after kidney and liver transplantation performed to correct factor h gene mutation. Am J Transplant 2005; 5: 1146-1150. PMID: 15816899.


11. Landau D, Shalev H, Levy-Finer G, Polonsky A, Segev Y, Katchko L. Familial hemolytic uremic syndrome associated with complement factor H deficiency. J Pediatr 2001; 138: 412-417. PMID: 11241053.


12. Nathanson S, Frémeaux-Bacchi V, Deschênes G. Successful plasma therapy in hemolytic uremic syndrome with factor H deficiency. Pediatr Nephrol 2001; 16: 554-556. PMID: 11465803.


13. Scoazec JY, Borghi-Scoazec G, Durand F, Bernuau J, Pham BN, Belghiti J, et al. Complement activation after ischemia-reperfusion in human liver allografts: incidence and pathophysiological relevance. Gastroenterology 1997; 112: 908-918. PMID: 9041253.


14. Bellamy MC, Gedney JA, Buglass H, Gooi JH. Complement membrane attack complex and hemodynamic changes during human orthotopic liver transplantation. Liver Transpl 2004; 10: 273-278. PMID: 14762866.


15. Rougier N, Kazatchkine MD, Rougier JP, Fremeaux-Bacchi V, Blouin J, Deschenes G, et al. Human complement factor H deficiency associated with hemolytic uremic syndrome. J Am Soc Nephrol 1998; 9: 2318-2326. PMID: 9848786.


Fig. 1
The plasma concentration of complement factor H analyzed by Western blot is 23% of normal value.
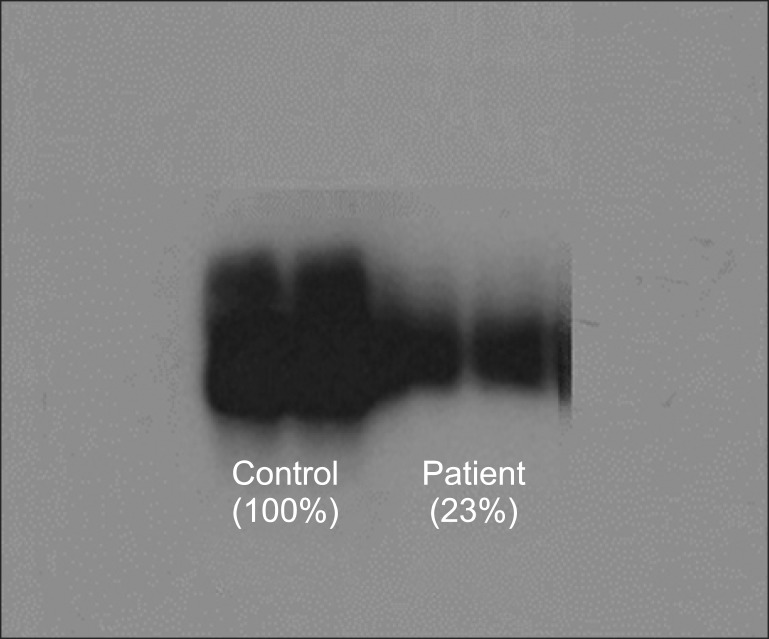
Table 2
Hemodynamic and Coagulation Profile during the Liver Transplantation
BP (S/M/D): blood pressure (systolic/mean/diastolic), HR: heart rate, CVP: central venous pressure, PT: prothrombin time, INR: international normalized ratio, aPTT: activated partial thromboplastin time, ACT: activated clotting time, CT: clotting time, CFT: clot formation time, MCF: maximum clot firmness, LI 60: lysis index amplitude at 60 min after clotting time, TEG: thromboelastography, MA: maximum amplitude.
- TOOLS