Effectiveness of milrinone for cardiogenic shock due to massive pulmonary aspiration: a case report
Article information
Abstract
Pulmonary aspiration of gastric contents is one of the most frightening complications during anesthesia. Although pulmonary aspiration of gastric contents in general surgical patients is not common and resulting long-term morbidity and mortality are rare, severe hypoxemia and other sequelae of pulmonary aspiration continue to be reported. We report a case of massive aspiration of gastric contents during induction of general anesthesia, resulting in cardiac arrest due to severe pulmonary hypertension and myocardial infarction. Sustained cardiac arrest and shock that did not respond the conventional resuscitation was successfully treated using milrinone. The patient was discharged without complications in 20 days.
Pulmonary aspiration of gastric contents is one of the most frightening complications during anesthesia. Although it has been reported that pulmonary aspiration of gastric contents in general surgical patients is not common and the resulting long-term sequelae are rare [1,2], fatal complications caused by pulmonary aspiration continue to be reported. Moreover, the practice of preoperative gastric decompression has changed and the preoperative application of a nasogastric tube is not guaranteed [3].
We experienced a case of severe pulmonary aspiration with cardiac arrest that did not respond to conventional resuscitation. We report on the benefit of milrinone in the resuscitation following massive aspiration and subsequent collapse.
Case Report
A 53-year-old man (170 cm, 61.3 kg) was admitted to our hospital with advanced gastric cancer. The patient did not eat any food for 10 days preoperatively. Abdominopelvic computed tomography and endoscopic findings indicated the presence of partial gastric outlet obstruction but the patient did not complain of nausea, vomiting or abdominal distension. The surgical team decided not to insert a nasogastric tube because the obstruction was partial and he had no obstructive symptoms.
On a pre-operative visit prior to the anesthesia, the patient disclosed no history of cardiovascular disease and did not report any chest pain or dyspnea. He was a current smoker at 35 pack-years. He had ceased drinking alcohol one year previously.
Elective distal gastrectomy was planned. After the administration of 150 mg of ranitidine premedication, the patient was transferred to operating room. He had some loose teeth and the anesthesiologist planned to conduct rapid sequence induction with a lightwand. After preoxygenation, 75 ug of fentanyl with 120 mg of propofol and 50 mg of rocuronium was administered. Endotracheal intubation with cricoids pressure was tried in 60 s and failed with a capnogram. After the failure of first trial of intubation, the patient was ventilated with facial mask 2-3 times. Abruptly massive vomiting developed with about 2 L of bilious vomitus. The patient was tilted in the Trendelenburg position and oral suction was done. Prompt tracheal intubation was performed and 700 ml of vomitus was aspirated via endotracheal tube. Oxygen saturation (SpO2) decreased to 80-85% and severe bradycardia converted to cardiac arrest. Sinus rhythm was restored immediately after cardiac compression and 1 mg of epinephrine. Arterial blood gas showed PaO2 of 56 mmHg and PaCO2 of 59 mmHg in FiO2 1.0 immediately after the endotracheal intubation and SpO2 increased to 99-100%. However, severe hypotension (< 50/30 mmHg) followed and the patient did not respond to a high dose of catecholamine and nitroglycerin infusion. Hypotension was sustained and bolus loading of 1 mg of epinephrine and chest compression was repeated 2-3 times. Electrocardiogram (ECG) showed sinus tachycardia with severe ST elevation and R-on-T phenomenon. A subclavian central venous catheter was secured. The central venous pressure (CVP) was 45 mmHg. With 1 mg of epinephrine, 0.5 mg/kg of milrinone was administered. Abruptly, CVP decreased to 18 mmHg and blood pressure was restored to 130/75 mmHg with a heart rate of 180 beats/min (bpm). Arterial PaO2 was 326 mmHg and PaCO2 was 48 mmHg. The ST elevation disappeared and changed to ST depression (-8.0 to -12.0 mm) with T inversion in lead II and V5. Three minutes after milrinone, hypotension developed again and 0.375 ug/kg/min of milrinone was started to infuse continuously with a low dose of epinephrine (0.02 ug/kg/min) and dobutamine (5 ug/kg/min). The vital signs were stable and further hypotensive or hypoxemic episodes did not develop. Intraoperative transesophageal echocardiography showed septal akinesia and ischemic changes in ECG were maintained. Oxygenation was excellent and there was no foamy, pinkish secretion or blood on endotracheal tube. However, a venoarterial extracorporeal membrane oxygenation (ECMO) was started at minimal flow in a concern of huge amount of aspiration.
The patient was transferred to coronary catheterization for the examination of the patency of coronary artery. There was acute myocardial infarction in distal right coronary artery with 99% eccentric occlusion. The lesion was old with the presence of collateral formation from the left anterior descending artery to the posterior division of right coronary artery. Percutaneous coronary intervention was performed with stent and balloon and the patient was transferred to intensive care unit. The patient showed stable hemodynamics. Milrinone and epinephrine were stopped and dobutamine (5 ug/kg/min) and nitroglycerin (0.2 ug/kg/min) were replaced. Radiographic examination of chest showed perihilar pulmonary edema, (Fig. 1) and there was considerable amount of red bean soup-colored secretion. The patient was ventilated in volume-controlled mode with 5 mmHg of positive end expiratory pressure. ECMO was applied at same minimal flow. In laboratory data, NT-proBNP was high (1,072 pg/ml) and other cardiac enzymes also increased (troponin T; 0.262 ng/ml, troponin I; 0.35 ng/ml). Intravenous antibiotics were administered but steroids were not used.
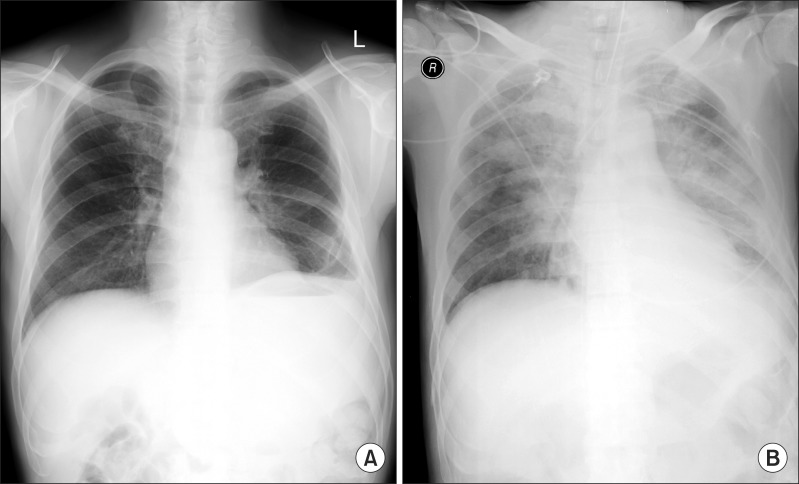
Radiographic examinations of chest revealed perihilar pulmonary edema immediately after transfer to ICU. (A) Preoperative chest PA, (B) Postoperative chest PA.
The day after the event, chest radiography revealed marked recovery and arterial blood gas was good. Cardiac enzymes had decreased. Two days after the event, the patient developed a fever of 38.2℃ and some consolidation in left lower lobe. However, he had alert mentality and good condition to extubate. Extubation was done and ECMO was removed. Five days after the event, C-reactive protein began to decrease and the patient was transferred to a ward. Thirteen days after the event, the patient had the planned elective operation and underwent palliative gastrojejunostomy due to peritoneal seeding. Twenty days after the event, the patient was discharged without complications.
Discussion
The damage mechanism of acid aspiration consists of two stages. Initially a direct chemical burn develops [1]. The damage of the alveolar endothelium results in impaired fluid removal from the alveolar space, and decreases of surfactant and pulmonary fibrosis. Frequently, it continues to lung edema, atelectasis or ventilation-perfusion mismatch (V/Q mismatch). Secondly, the endothelial dysfunction promotes the inflammatory process and platelet aggregation and inhibits the secretion of vasodilating neurotransmitters of nitrous oxide or prostacyclin causing pulmonary vasoconstriction [4]. Moreover, arterial hypoxemia also induces hypoxic pulmonary vasoconstriction [5]. Rapid increases in pulmonary vascular resistance can increase right ventricular (RV) afterload and dilation, decreasing the left ventricular (LV) preload and cardiac output [2,6]. The right coronary perfusion decreases because of increased RV wall tension. RV ischemia may occur as a result of decreased right coronary perfusion and increased RV overload. It is easier if there is a prior coronary arterial stenotic lesion. Acute pulmonary hypertension and right ventricular failure (RVF) can cause cardiogenic shock or sudden arrest [6]. Cardiac arrest and myocardial infarction related to the pulmonary aspiration are rare complications [7]. Presently, the resulting cardiogenic shock that did not respond to routine resuscitation and responded to milrinone was very interesting. In this case, the patient was healthy without chest pain or dyspnea but he had silent coronary artery disease with prior collateral formation.
The clinical course of pulmonary aspiration of gastric contents during anesthesia varies widely. In up to 50% of the patients who are aspirated, there are no consequences or only mild events. Warner et al. [2] suggested that patients with clinically apparent aspiration who do not develop symptoms within 2 hours are unlikely to have respiratory sequelae. However, pulmonary aspiration could induce severe hypoxemia due to pulmonary edema, bronchopneumonia and the development of adult respiratory distress syndrome. Pulmonary aspiration occurs mainly during induction and emergent operation, insufficient fasting and improper anesthesia are predisposing factors in the general surgical patients [7,8].
The risk of pulmonary aspiration in general surgical patients depends on the combination of volume and pH of gastric contents. The critical points are 0.4 ml/kg at pH < 2.5 [1]. To reduce the risk of pulmonary aspiration, there are some preventive strategies to reduce the volume and acidity of gastric contents. There is no evidence that pharmacological preventions using H2 antagonists or proton pump inhibitors reduce pulmonary aspiration. These medications are effective and inexpensive methods to reduce the acidity of gastric contents, but chemoprophylaxis is not perfect because gastric bile is not affected by these agents and induces a worse pneumonitis than gastric acid [8,9]. In this case, the patient was administered preoperative H2 antagonist. Although we applied emergent medicines and preventive ECMO, the clinical course was very short and successful despite the huge amount of bilious vomitus. Prior chemophylaxis was considered to be helpful.
It is important to decrease the volume of gastric contents. Sufficient starvation and preoperative gastric decompression is needed. The amount of gastric contents is difficult to estimate. Most clinicians usually judge the amount of gastric contents from the physical examinations and the presence of obstructive symptoms like nausea, vomiting or abdominal distension. In this case, surgical team decided not to apply nasogastric tube because the patient had prolonged preoperative starvation period and did not show any obstructive symptoms. Current concerns about the insertion of nasogastric tube include ineffectiveness for prevention of aspiration and its potential risks. Recent extensive studies showed that the effects of preoperative nasogastric decompression are controversial and did not change the incidence of pulmonary aspiration, even in emergency cases [10]. However, special precautions about full stomach should be necessary even in elective patients without obstructive symptoms. The position of the tumor and the possibility of obstruction should be examined carefully. Gastrointestinal lesions obstructing partially or completely significantly increase a risk of pulmonary aspiration of gastric contents. Insertion of nasogastric tube is a very effective method to reduce gastric volume in patients at high risk for aspiration.
Initial management of pulmonary aspiration of gastric contents is that the head down position and the oropharyngeal suction should be done. After rapid sequence intubation with cricoids pressure, 100% oxygen must be applied and an excessive hypercapnia should be avoided to reduce the hypoxic pulmonary vasoconstriction [8]. Sudden cardiac arrest is a rare complication. In our case, the patient did not respond to routine cardiopulmonary resuscitation steps. The sinus rhythm returned quickly but cardiogenic shock was not recovered and several cardiac arrests were repeated. The vasodilating property of dobutamine and nitroglycerin and high dose epinephrine did not solve the problem of acute pulmonary hypertension and following RVF. Hypoxemic and hypercapnic event caused by massive pulmonary aspiration resolved soon but repeated cardiac arrest with elevated CVP (45 mmHg) means ischemic heart failure or pulmonary hypertension-induced RVF. We initially used intravenous milrinone to relieve pulmonary hypertension. If there was no improvement, we should consider myocardial infarction and move to coronary catheterization. Fortunately, vital signs were immediately restored and CVP decreased to normal. In this case, the patient had chronic coronary heart disease involving right coronary artery. The lesion already had collateral circulations. He had no chest pain and no dyspnea. It would be a trigger of cardiac arrest related to pulmonary hypertension due to massive aspiration. However, it was not persuasive that ischemia-induced acute RVF was the cause of persistent cardiogenic shock.
Among pulmonary vasodilators, the classical selective pulmonary vasodilator is inhaled nitric oxide (INO), but INO needs a specialized device to apply and 30% of patients with pulmonary hypertension are INO non-responders [11]. Milrinone is a non-glycosidic, non-sympathomimetic drug that increases myocardial and vascular smooth muscle cyclic adenosine monophosphate concentrations by inhibiting phosphodiesterase fraction III enzymes, thus augmenting intracellular calcium concentrations with resultant increased myocardial contractility and pulmonary and systemic vasodilation [12]. Due to the lack of selectivity for the pulmonary vasculature, milrinone is accompanied by a profound systemic hypotension in therapeutic concentrations of the drug. Haraldsson et al. [12] suggested that inhalation of milrinone selectively dilates the pulmonary vasculature without systemic effects in cardiac surgical patients with pulmonary hypertension. Moreover, inhaled milrinone potentiates and prolongs the pulmonary selective vasodilatory effect of prostacyclin. However, during resuscitation condition, the use of intravenous milrinone has several benefits including intravenous availability, absence of the need to prepare specialized delivery systems and monitoring and excellent potency compared to those of the traditional vasodilators like nitrates or prostaglandins or inhaled nitric oxide or inhaled milrinone [13].
Venoarterial ECMO is an effective rescue method for cardiopulmonary failure in which all pharmacologic and ventilator supports are ineffective [14]. However, the use of ECMO is invasive and more expensive. It induces bleeding complications [14]. In the 2010 revised international consensus of cardiopulmonary resuscitation [15], there was no recommendation of the administration of potent vasodilators like milrinone in severe pulmonary hypertensive episodes.
In conclusion, the use of milrinone might be an effective option in the patients with cardiogenic shock with pulmonary aspiration of gastric contents who did not respond the traditional advanced cardiac life support guidelines.