Systemic amyloidosis is rare and usually underdiagnosed. Misfolded protein fibrils deposit into tissues in various organs. Systemic amyloidosis has subtypes of light chain (AL) amyloidosis, amyloid A (AA) amyloidosis, dialysis-related amyloidosis, hereditary amyloidosis such as familial amyloidosis with polyneuropathy, cardiac or renal amyloidosis, and organ specific amyloidosis such as Alzheimer disease, cardiac, laryngeal, or pulmonary amyloidosis [1]. AL amyloidosis is the most common subtype and is caused by deposition of protein derived from immunoglobulin light chain fragments. Laryngeal or pulmonary involvement requires attention by the anesthetist to airway management in the perioperative period [2,3]. Therefore, diagnosis of amyloidosis for any surgical patient should prompt an evaluation of the involvement of other organs, including the airway, before anesthetic induction and complete preparation for difficult airway management. We present a case of systemic amyloidosis patient whose diagnosis occurred subsequent to anesthesia.
Case Report
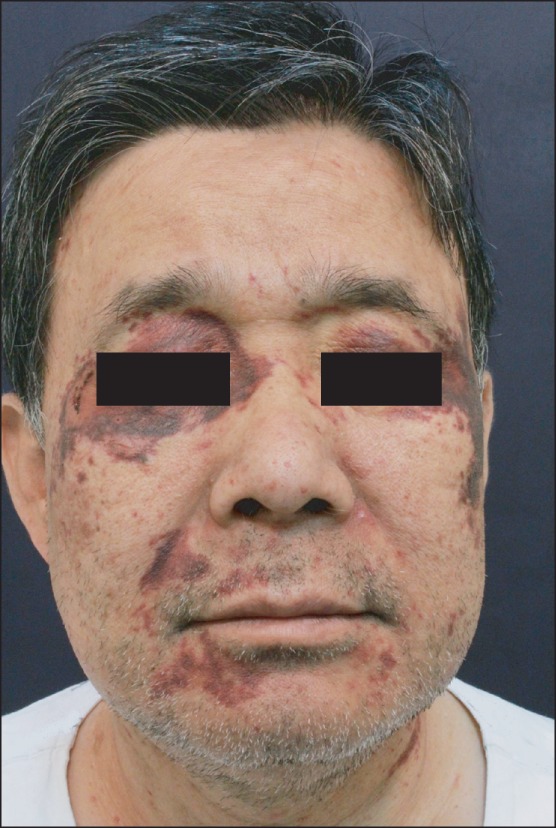
A 63-year-old male, 159 cm in height and 70 kg in weight, was scheduled to undergo resection of cervical epidural mass. The patient had a medical history of well-controlled hypertension and old healed pulmonary tuberculosis. The patient had facial and neck purpuras and ecchymoses, whose cause could not be identified at a private dermatologic clinic. Blood test results concerning platelet count, prothrombin time and activated partial thrombosplastin time were normal.
General anesthesia was induced with propofol 100 mg and remifentanil 0.5 ┬Ąg/kg/min. Rocuronium 50 mg was administrated to facilitate tracheal intubation. Anesthesia was maintained with 6 vol% desflurane with 50% O2 in air, remifentanil, and rocuromium. After a change from a supine to a prone position, bleeding from the lacerated facial purpuras was recognized while identifying head position on a Mayfield headrest. Swollen purpuras and eyeballs hyperemia were evident. The operation was canceled in order to evaluate the facial lesions and the patient was transported to the postanesthetic care unit when fully conscious.
Skin biopsy from the facial lesions (Fig. 1) and serum free light chain assay were performed during the stay at the general ward, and resulted in the diagnosis of systemic amyloidosis of monoclonal gammopathy with lambda type. Multiple myeloma, which usually accompanies systemic amyloidosis, was excluded after bone marrow biopsy and immunoprotein measurement of serum and urine.
The patient had complained of dyspnea since post-anesthesia day 16. Bilateral pleural effusion and interstitial pulmonary edema were identified by high resolution chest computed tomography. Pulmonary function test revealed moderate degree of chronic obstructive pulmonary disease. Cervical magnetic resonance imaging taken 48 days after the anesthesia showed spontaneous resolution of the epidural mass. Post-anesthetic echocardiograph showed thickened myocardium, which was not confirmatory of cardiac amyloidosis. According to abdominal ultrasound, there was no hepatomegaly or splenomegaly. Symptoms and signs suggesting the involvement of other organs, such as renal, neurologic, and hematologic abnormalities, were not observed. Treatment with chemotherapy and auto-peripheral blood stem cell transplantation was planned.
Discussion
Systemic amyloidosis is caused by tissue deposition of insoluble misfolded protein fibrils. AL amyloidosis, the most common type of systemic amyloidosis, is characterized by deposition of protein derived from immunoglobulin light chain fragments. AL amyloidosis is caused by the monoclonal expression of plasma cells in the bone marrow that secrete a clonal immunoglobulin light chain of kappa or lambda type, which deposits as amyloid fibrils in tissues [1].
Clinical manifestations are determined by the type of amyloid and the distribution of deposition. AL amyloidosis syndromes include renal involvement with nephrotic range proteinuria or renal failure; cardiomyopathy with thick-walled heart, low voltage on electrocardiogram, and pericardial and pleural effusions; cholestatic hepatopathy; peripheral neuropathy and autonomic neuropathy; infiltration of soft tissues, of which macroglossia is a pathognomonic findings; and purpura, including periorbital ecchymoses due to capillary involvement and/or clotting factor deficiency [4].
Any patient presenting with cardiomyopathy with preserved systolic function, heavy albuminuria, an unexplained sensorimotor peripheral neuropathy, hepatomegaly, or atypical monoclonal gammopathy of undetermined significance or myeloma should be suspected of having AL amyloidosis [5]. But, patients may have nonspecific symptoms like dyspnea, fatigue, weight loss, edema, paresthesia, diarrhea or constipation due to autonomic dysfunction, dry mouth, or hoarseness, which usually present in more common disorders.
Once amyloid is identified by biopsy, further evaluation for systemic disease and organ involvement should include creatinine, 24 hour urine total protein, electrocardiogram, echocardiogram, and alkaline phosphatase. Cardiac biomarkers like troponin and N-terminal pro-brain natriuretic peptide should be measured because the extent of cardiac involvement is important for determining prognosis [6].
All patients diagnosed as systemic AL amyloidosis need therapy. Early diagnosis provides much broader therapeutic options and enables reversal of organ damage. Treatment is aimed to eliminate misfolded amyloidogenic light chains, minimize treatment toxicity, and support the targeted organ function. Treatment should be individualized based on age, organ dysfunction, and regimen toxicity. Therapeutic options are high-dose melphalan/autologous stem cell transplantation, corticosteroids, alkylating agents, immunomodulatory drugs, and proteasome inhibitors [5].
Cardiac amyloidosis is caused by extracellular deposition of amyloid in the endomyocardium, conduction pathway or coronary vessels. Symptoms may vary from atypical symptoms like fatigue, lightheadedness, and pedal edema to arrhythmia or ischemic symptoms, and even heart failure. Cardiac amyloidosis can be diagnosed directly by endomyocardial biopsy or indirectly by echocardiography. Evaluating cardiac involvement is important to determine the prognosis of systemic amyloidosis. There was no typical finding indicative of cardiac amyloidosis like granular sparkling appearance of myocardium in an echocardiography that is performed preoperatively. Interpretation of an echocardiography performed after the diagnosis of AL amyloidosis includes thickened and speckled myocardium, without obvious cardiac involvement. Presently, there was an incomplete left bundle branch block on preoperative electrocardiogram; however, this finding and echocardiographic result complicated the diagnostic exclusion of cardiac amyloidosis. Laryngeal amyloidosis is rare but may be caused by either systemic or localized amyloidosis. Patients most commonly present with hoarseness, but may also have stridor, dyspnea, or dysphagia. Systemic involvement should be ruled out after the laryngeal lesion is confirmed as amyloidosis by tissue biopsy. Treatment of localized laryngeal amyloidosis is surgical resection and the long-term outcome is excellent. A case of anesthetic management with a patient with laryngeal amyloidosis has been reported [7].
Pulmonary amyloidosis includes tracheobronchial infiltration, persistent pleural effusions, parenchymal nodules, and, rarely, pulmonary hypertension. Tracheobronchial infiltration may cause hoarseness, stridor, airway obstruction, and dysphagia. One case report described the extensive involvement in the entire laryngo-tracheo-bronchial tree [2]. In the report, a 53-year-old man had been followed up for five years for systemic amyloidosis involving the larynx, trachea, and bronchi. Ventilation was impossible with airway obstruction after intubation, which was relieved by extensive debridement of tissues in the lower trachea using a rigid bronchoscope under supraglottic jet ventilation.
Precaution is imperative when a patient with laryngeal or tracheal amyloidosis involvement during anesthetic induction. The mass may cause airway obstruction, increasing the risk during intubation. Imaging is required before induction to evaluate the extent of obstruction. A differential diagnosis is needed, such as a malignant tumor of the airway. Unfortunately, the systematic amyloidosis was not diagnosed in our patient prior to anesthetic induction. If amyloidosis were diagnosed beforehand, evaluation for airway involvement should have been performed before anesthesia. Preoperative airway assessment is recommended to avoid any complications such as airway obstruction due to mass, desaturation, and even death [3]. Maintaining spontaneous ventilation without muscle relaxant may be considered as appropriate technique during induction of patients with airway involvement. It is recommended that fiberoptic bronchoscopy and tracheostomy be prepared in case of difficult airway. Also, various sizes of tracheal tubes should be prepared. Airway obstruction can be a serious problem. The patient should be monitored in the intensive care unit postoperatively because obstruction can occur at emergence and extubation.
In our case, relevant symptoms that would definitively rule out systemic amyloidosis were typical purpura and ecchymoses in face and neck, without obvious coagulation defect. Purpura in a periorbital distribution in amyloidosis patients, also described as raccoon eyes, is quite characteristic of AL amyloidosis. Hemorrhagic lesions as a mucocutaneous manifestation are caused by deposition of amyloid in and around vessels, resulting in increased vascular fragility. Presently, it was very fortunate that the patient with just skin lesions did not have involvement of other critical organs, including the heart, kidney and especially the larynx.
Systemic amyloidosis especially involving the airway is rare but anesthesiologists should recognize the possibility during the perioperative period. Patient's airway should be evaluated while formulating the anesthetic plan as well as the function of the involved organs, and provisions should be in place to manage airway difficulties.