 |
 |
|
|
Abstract
Background
We investigated the protective effects of propofol in the HK-2 cell line of human kidney proximal tubular cells against hydrogen peroxide (H2O2)-induced oxidative stress.
Methods
After pretreatment with different concentrations of propofol (0 µM, 10 µM, 25 µM and 50 µM) for 30 minutes, HK-2 cells were exposed to 8 mM H2O2 for 4 hours. Cell death was assessed by measuring the percentage of lactate dehydrogenase (LDH) release and by counting viable cells. The nature of cell death was assessed by doubles-taining cells with fluorescein isothiocyanate-labeled Annexin V and propidium iodide, and then analyzing the cells using flow cytometry.
Results
After exposure to 8 mM H2O2 for 4 hours, the percentage of LDH release was 45.1 ± 4.2% and the number of viable HK-2 cells was 5.2 ± 6.0%. Pretreatment with propofol suppressed H2O2-induced LDH release in a concentration-dependent manner, reducing the percentage of LDH release to 38.1 ± 5.6%, 33.5 ± 6.3%, and 26.2 ± 3.8% of the controls at 10 µM, 25 µM and 50 µM propofol, respectively. Numbers of viable cells increased following propofol pretreatment, with 11.4 ± 10.9%, 19.5 ± 16.1%, and 32.4 ± 23.3% cell survival rates after pretreatment with 10 µM, 25 µM and 50 µM propofol, respectively. Analyses of flow cytometry showed that the propofol pretreatment decreased the percentage of necrotic and late apoptotic cells.
Renal ischemia/reperfusion (I/R) damage can occur in many clinical situations such as during reno-vascular surgery, clamping of the aorta, shock, trauma, and renal transplantation. Acute tubular necrosis (ATN) and renal failure related to I/R are the main reasons for morbidity and mortality in hospitalized patients [1]. ATN arises as a result of damage created by free oxygen radicals that are formed during I/R [2]. Oxygen free radicals, such as superoxide anion, hydroxyl radicals and hydrogen peroxide (H2O2) are key mediators of renal reperfusion injury [3]. These reactive oxygen species cause lipid peroxidation of the renal cell membrane, which results in intracellular calcium overload and subsequent necrotic cell death [4,5].
Following renal transplantation, exposure of the graft to variable levels of I/R damage delays graft function, and can even cause graft loss [6]. Hence, approaches that prevent I/R damage should promote graft functions and enhance graft lifetime. When any manipulation that involves a risk of renal ischemic damage is planned, it would be prudent to have prophylactic treatments or anesthetic techniques in place in order to avoid I/R damage.
Propofol (2, 6-diisopropylphenol), a highly lipid-soluble anesthetic, is widely used for the induction and maintenance of general anesthesia. Propofol ameliorates I/R injury in several organs, including the heart [7], lungs [8], brain [9], liver [10], and testicles [11]. Some reports have described a protective effect of propofol against oxidative stress in rats at the nephrocyte [12] and whole-animal levels [13]. However, there have been few reports on the effects of propofol on oxidative stress in human kidney cells.
In this study, we used the HK-2 immortalized human proximal tubular cell line as a culture model, because proximal tubular cells are known to be the cell type most susceptible to ischemia in the kidney [14]. Moreover, HK-2 cells have been used to study renal physiology and pathophysiology in vitro [15]. We hypothesized that propofol would protect human kidney cells against oxidant-mediated damage. To test this hypothesis, we injured HK-2 cells with H2O2 and assessed cell death by measuring lactate dehydrogenase (LDH) release, counting viable cells, and performing flow cytometry on fluorescein isothiocyanate (FITC)-labeled Annexin V/propidium iodide (PI) double-stained cells.
Immortalized human proximal tubular cells of the HK-2 cell line (American Type Culture Collection, Manassas, VA, USA) were grown and passaged in 25-cm2 cell culture flasks containing culture medium (keratinocyte serum-free medium with 5 ng/ml epidermal growth factor and 40 mg/ml bovine pituitary extract; Gibco BRL, NY, USA) and antibiotics (100 U/ml penicillin G, 100 µg/ml streptomycin and 0.25 µg/ml amphotericin B). Cultured cells were incubated at 37℃ in a 100% humidified incubator containing 5% CO2. Cells were plated in 24-well plates at 80% confluence, and culture medium was replaced every 2 days. Three days after plating, HK-2 cells were challenged with 8 mM H2O2 (Sigma Chemical, St. Louis, MO, USA) diluted in serum-free medium containing different concentrations of propofol (Tocris Chemical Co, Ellisville, MO, USA) and incubated for 4 hours.
Depending on the groups, cells were incubated with 0 µM, 10 µM, 25 µM, or 50 µM propofol for 30 minutes and then incubated for a further 4 hours after the addition of H2O2 to a final concentration of 8 mM H2O2. Propofol was dissolved in dimethyl sulfoxide (DMSO; Sigma Chemical, St. Louis, MO, USA), yielding a final DMSO concentration in media of 0.0025-0.0125%. Tests showed that DMSO over this concentration range had no effect on oxidative stress or cell viability (data not shown).
Whereas LDH normally localized to the cytoplasm in HK-2 and most other cell types, its release in response to cell damage is a widely used indicator of incipient cell death. To quantify cell death, we measured LDH enzymatic activity in the collected medium using the spectrophotometric CytoTox 96®Non-Radioactive Cytotoxicity Assay (Promega, Madison, WI, USA), and then calculated the percentage of LDH release. The percentage of LDH release was calculated as (LDH)test / (LDH)total × 100, where (LDH)test is the measured LDH level in the experimental medium and (LDH)total is the total cellular LDH level per well measured after killing all cells by addition of the lysis solution (1% Triton X-100) provided as part of the kit.
Cell viability was measured using the trypan blue dye exclusion method. After treatment protocols were performed, cells were harvested and stained with 0.4% trypan blue dye for 5 minutes. The number of viable cells and total cells were counted using a hemocytometer.
The number of remaining nonviable cells was expressed as a percentage of the total number of cells.
Cell death was further analyzed by double staining cells with FITC-labeled Annexin V and PI using the Annexin V-FITC apoptosis detection kit (BD Biosciences, San Jose, CA, USA). Both floating and adherent cells were collected and plated onto a 12-well plate at 1 × 106 cells/well. Cells were washed with phosphate buffered saline (PBS) and collected by trypsinization. After centrifugation at 400 × g for 5 minutes at 4℃, pelleted cells were washed twice with cold PBS and then resuspended in Annexin V binding buffer. A total of 100 µl of cell suspension was transferred to test tubes, to which 5 µl of FITC-labeled Annexin V and 5 µl of PI were added. The cells were gently vortexed and incubated for 15 minutes at room temperature in the dark. After adding 200 µl of binding buffer to each tube, the cells were analyzed by flow cytometry using the FACSCalibur system (BD Biosciences, San Jose, CA, USA). Annexin V-FITC and PI emissions were detected in the FL 1 and FL 2 channels using 525 and 575 nm emission filters respectively. The Annexin V-FITC-negative/PI-negative population was considered to include all normal healthy cells. Annexin V-FITC-positive/PI-negative cells were regarded as a measure of early apoptosis. Annexin V-FITC-positive/PI-positive population was considered to represent late apoptotic or necrotic cells, and Annexin V-FITC-negative/PI-positive cells were considered to include necrotic cells [16]. The percentage distributions of normal, early apoptotic, late apoptotic, and necrotic cells were calculated using ModFitLT V3.0 software (BD Biosciences, San Jose, CA, USA).
Statistical analyses were performed using the SigmaStat program (version 3.10; Systat software, Chicago, IL, USA). Multiple groups were compared using one-way analysis of variance (ANOVA) or Kruskal-Wallis one-way ANOVA on Ranks followed by pairwise multiple comparisons using a Tukey post-hoc test as appropriate. In all comparisons, a P value less than 0.05 was considered to indicate a statistically significant difference. Data are presented as mean ± SD unless stated otherwise.
Treatment of HK-2 cells for 4 hours by exposure to concentrations of 3 mM, 5 mM, or 8 mM H2O2 or lysis solution (control group) increased the percentage of LDH release by 19.0 ± 2.2%, 22.4 ± 8.8%, 41.2 ± 6.5%, and 100.0 ± 7.4%, respectively (data not shown). We chose to use 8 mM H2O2 in subsequent experiments as it induced an appropriate level of oxidative challenge. As shown in Fig. 1, propofol treatment significantly decreased the amount of LDH released at 8 mM H2O2. Compared with a rate of LDH release of 45.1 ± 4.2% (n = 8) without propofol pretreatment, the rate of LDH release after pretreatment with 10 µM propofol was 38.1 ± 5.6%, (n = 8, P < 0.05), pretreatment with 25 µM propofol caused LDH release at a rate of 33.5 ± 6.3% (n = 8, P < 0.05), and pretreatment with 50 µM propofol caused LDH release at a rate of 26.2 ± 3.8% (n = 8, P < 0.05).
The rate of LDH release after pretreatment with 50 µM propofol was significantly different from that following pretreatment with either 10 µM or 25 µM of propofol (P < 0.05) and there was no significant difference between rates of LDH release after pretreatment with 10 µM propofol and pretreatment with 25 µM propofol (Fig. 1).
After treatment of HK-2 cells with 8 mM H2O2 for 4 hours, 5.2 ± 6.0% (n = 20) of HK-2 cells were viable (control group). When HK-2 cells were treated with 25 µM and 50 µM propofol 30 minutes before exposure to 8 mM H2O2 and then incubated in the presence of 8 mM H2O2 for 4 hours, the percentages of viable cells significantly increased to 19.5 ± 16.1% (n = 20), and 32.4 ± 23.3% (n = 20), respectively (P < 0.05 when compared with the control group, Fig. 2). The percentage increase in cell viability after pretreatment with 50 µM propofol was significantly different from that after pretreatment with either 10 µM or 25 µM propofol (P < 0.05) and there was no significant difference in cell viability following pretreatment with either 10 µM or 25 µM propofol (Fig. 2).
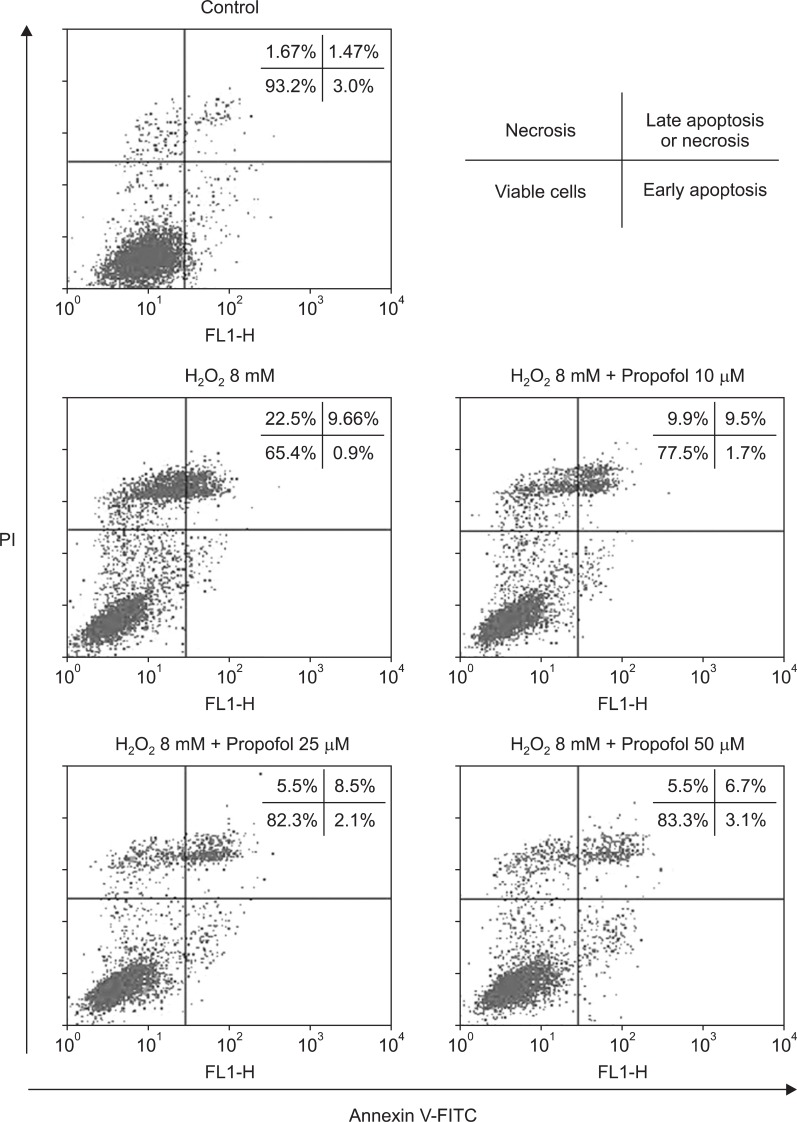
Annexin V-FITC binding analysis and PI staining were performed to quantify cell death arising from apoptosis and necrosis, respectively, with all of the experiments performed five times. Use of FACS to analyze, trypsinized cells revealed that the respective percentages of intact Annexin V-FITC-negative/PI-negative cells from the control and H2O2 group were 93.2 ± 1.0% and 65.4 ± 5.9% of the two populations. The numbers of intact cells were significantly increased (P < 0.05) in the propofol groups, with respective percentages after pretreatment with 10 µM, 25 µM, and 50 µM propofol of 77.5 ± 1.4%, 82.3 ± 2.0%, and 83.3 ± 2.7%. As shown in Fig. 3, pretreatment with propofol significantly decreased (P < 0.05) the number of Annexin V-FITC-negative/PI-positive cells. Respective percentages of these cells after pretreatment with 10 µM, 25 µM, and 50 µM propofol were 9.9 ± 0.6%, 5.5 ± 0.8%, and 5.5 ± 0.7% compared with cells treated with H2O2 alone (22.5 ± 3.7%). The numbers of annexin V-FITC-positive/PI-positive cells were significantly lower (P < 0.05) in groups pretreatment with either 25 µM or 50 µM propofol (respective value of 8.5 ± 0.4% and 6.7 ± 0.6%) compared to the H2O2 group (9.7 ± 0.6%). Dead cells were defined by the sum of the numbers of Annexin V-FITC-negative/PI-positive cells and annexin V-FITC-positive/PI-positive cells. As shown in Fig. 3, the percentages of dead cells were significantly lower (P < 0.05) in cells from the propofol groups (respective values of 19.4 ± 0.9%, 14.0 ± 0.3%, 12.2 ± 1.0% for cells pretreated with 10 µM, 25 µM, and 50 µM propofol) than in cells from the H2O2 group (32.2 ± 0.5%).
In our present study, we evaluated whether propofol has a beneficial effect against renal I/R injury in human cells. The results indicated that propofol protects human proximal tubular cells against H2O2-induced oxidative stress in vitro. Dealing with the detrimental effects of renal I/R injury that can result in acute renal failure, remains a serious clinical challenge [17]. Oxygen free radicals are key mediators of renal reperfusion injury [3]. During the post-ischemic reperfusion period, reactive oxygen species, such as the superoxide anion, hydroxyls radical, and H2O2, are generated. These reactive oxygen species cause lipid peroxidation of the renal cell membrane, resulting in intracellular calcium overload and subsequent necrotic cell death [4,5]. In this study, we used H2O2-mediated oxidative injury of HK-2 cells as an in vitro model of reperfusion injury. We selected H2O2 as the source of oxidative stress in this experiment because it is a relatively stable oxidative stress radical that causes a variety of cell and tissue injuries. We used the HK-2 human renal proximal tubular cell culture model because renal cells are susceptible to H2O2-induced oxidative damage and renal cells with proximal tubular characteristics are much more susceptible to I/R injury than those with distal tubular characteristics [18]. Moreover, HK-2 cells are used to study in vitro renal physiology and pathophysiology [15]. In our preliminary H2O2 oxidative stress challenge tests using these cells, we found that a 4-hour exposure to 5 mM H2O2 resulted in an LDH release of 22.4 ± 8.8%, similar to the previously reported value of 25% after a 4-hour challenge with 5 mM H2O2 [19].
Many reports have demonstrated that some anesthetic agents can attenuate oxidative stress in the kidney. Sevoflurane has anti-inflammatory effects and anti-necrotic effects in H2O2-treated HK-2 cells [19]. Propofol, which is chemically similar to a phenol-based free radical scavenger [20], protects against oxidative stress in renal I/R injury [12]. Both of these studies involved measuring levels of serum creatinine and blood urea nitrogen and histological analysis of I/R injury in rat models of kidney damage. Histological findings from the two studies showed that propofol significantly reduced tissue necrosis. Another report, which suggested that propofol has antioxidant effects following kidney transplantation in humans, examined the impact of renal transplantation on oxidative stress and inflammation by measuring changes in the level of 8-iso-PGF2α and 15-keto-dihydro-PGF2α, respectively [21]. The same study compared the antioxidant capacities of propofol and thiopentone during transplantation and after surgery. The levels of 8-iso PGF2α were significantly lower in the propofol group than in the thiopentone group [21]. However, propofol and thiopentone were used as induction agents in these experiments, not as maintenance agents. The plasma concentrations of propofol are reportedly 40-60 mM at the time of induction of anesthesia, and 10-25 mM during the maintenance of anesthesia [22]. To date, reports on the protective effects of propofol at the maintenance dose and studies on human renal cells are rare. Therefore we chose propofol concentrations of 10-50 mM, spanning the maintenance-dose range, to determine the clinical relevance of the protective effects of propofol. Pretreatment with 50 mM propofol in a sample group was significantly different from 10 µM or 25 µM of propofol in another sample group in the experiments measuring LDH release and viable cell count, but not in flow cytometric analysis. There were many other causes for that difference, one of which was that more figures from future data might be needed for accurate flow cytometric study.
It is possible that the concentration of propofol that produces antioxidant effects may differ in a tissue and species-specific manner. Some authors have reported no beneficial effect of propofol in cardiac surgical patients when administrated at a conventional clinical concentration of 2-4 mg/ml (11-23 mM) compared with the volatile anesthetic sevoflurane [23]. In contrast, propofol, when given at 6-8 mg/ml (35-46 mM) during myocardial ischemia and the early phase of reperfusion, attenuates free-radical-mediated and inflammatory components of myocardial reperfusion injury in patients undergoing elective coronary artery bypass graft surgery more effectively than the volatile anesthetic isoflurane [24]. These observations suggest that the potential cardioprotective effects of propofol in patients are dose-dependent. Divergent effects of propofol have also been reported with respect to vascular endothelial cells [25].
In conclusion, propofol attenuates renal oxidative injury in vitro, which has potential clinical implications. Thus, propofol may be a good anesthetic agent in surgeries that carry a risk of renal I/R injury, such as kidney transplantation. However, further in vivo and in vitro studies are needed to elucidate the protective mechanism involved and to establish the optimally protective concentration range of propofol in humans.
Acknowledgements
This study was supported by a grant (2005-177) from the Asan Institute for Life Sciences, Seoul, Korea.
References
1. Lieberthal W, Nigam SK. Acute renal failure. I. Relative importance of proximal vs. distal tubular injury. Am J Physiol 1998; 275: F623-F631. PMID: 9815122.


2. Paller MS, Hoidal JR, Ferris TF. Oxygen free radicals in ischemic acute renal failure in the rat. J Clin Invest 1984; 74: 1156-1164. PMID: 6434591.



3. Paller MS, Neumann TV. Reactive oxygen species and rat renal epithelial cells during hypoxia and reoxygenation. Kidney Int 1991; 40: 1041-1049. PMID: 1662318.


4. Salahudeen AK. Role of lipid peroxidation in H2O2-induced renal epithelial (LLC-PK1) cell injury. Am J Physiol 1995; 268: F30-F38. PMID: 7840245.


5. Sheridan AM, Fitzpatrick S, Wang C, Wheeler DC, Lieberthal W. Lipid peroxidation contributes to hydrogen peroxide induced cytotoxicity in renal epithelial cells. Kidney Int 1996; 49: 88-93. PMID: 8770953.


6. Peeters P, Vanholder R. Therapeutic interventions favorably influencing delayed and slow graft function in kidney transplantation: mission impossible? Transplantation 2008; 85(7 Suppl): S31-S37. PMID: 18401261.


7. Lim KH, Halestrap AP, Angelini GD, Suleiman MS. Propofol is cardioprotective in a clinically relevant model of normothermic blood cardioplegic arrest and cardiopulmonary bypass. Exp Biol Med (Maywood) 2005; 230: 413-420. PMID: 15956771.


8. Balyasnikova IV, Visintine DJ, Gunnerson HB, Paisansathan C, Baughman VL, Minshall RD, et al. Propofol attenuates lung endothelial injury induced by ischemia-reperfusion and oxidative stress. Anesth Analg 2005; 100: 929-936. PMID: 15781500.


9. Ergun R, Akdemir G, Sen S, Tasci A, Ergungor F. Neuroprotective effects of propofol following global cerebral ischemia in rats. Neurosurg Rev 2002; 25: 95-98. PMID: 11954772.


10. Wang H, Xue Z, Wang Q, Feng X, Shen Z. Propofol protects hepatic L02 cells from hydrogen peroxide-induced apoptosis via activation of extracellular signal-regulated kinases pathway. Anesth Analg 2008; 107: 534-540. PMID: 18633031.


11. Unsal A, Devrim E, Guven C, Eroglu M, Durak I, Bozoklu A, et al. Propofol attenuates reperfusion injury after testicular torsion and detorsion. World J Urol 2004; 22: 461-465. PMID: 15580508.


12. Wang HH, Zhou HY, Chen CC, Zhang XL, Cheng G. Propofol attenuation of renal ischemia/reperfusion injury involves heme oxygenase-1. Acta Pharmacol Sin 2007; 28: 1175-1180. PMID: 17640480.


13. Yuzbasioglu MF, Aykas A, Kurutas EB, Sahinkanat T. Protective effects of propofol against ischemia/reperfusion injury in rat kidneys. Ren Fail 2010; 32: 578-583. PMID: 20486841.


14. Ryan MJ, Johnson G, Kirk J, Fuerstenberg SM, Zager RA, Torok-Storb B. HK-2: an immortalized proximal tubule epithelial cell line from normal adult human kidney. Kidney Int 1994; 45: 48-57. PMID: 8127021.


15. Racusen LC, Monteil C, Sgrignoli A, Lucskay M, Marouillat S, Rhim JG, et al. Cell lines with extended in vitro growth potential from human renal proximal tubule: characterization, response to inducers, and comparison with established cell lines. J Lab Clin Med 1997; 129: 318-329. PMID: 9042817.


16. Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods 1995; 184: 39-51. PMID: 7622868.


17. Aronson S, Blumenthal R. Perioperative renal dysfunction and cardiovascular anesthesia: concerns and controversies. J Cardiothorac Vasc Anesth 1998; 12: 567-586. PMID: 9801983.


18. Weinberg JM, Buchanan DN, Davis JA, Abarzua M. Metabolic aspects of protection by glycine against hypoxic injury to isolated proximal tubules. J Am Soc Nephrol 1991; 1: 949-958. PMID: 1883965.


19. Lee HT, Kim M, Jan M, Emala CW. Anti-inflammatory and antinecrotic effects of the volatile anesthetic sevoflurane in kidney proximal tubule cells. Am J Physiol Renal Physiol 2006; 291: F67-F78. PMID: 16478975.


20. Green TR, Bennet SR, Nelson VM. Specificity and properties of propofol as an antioxidant free radical scavenger. Toxicol Appl Pharmacol 1994; 129: 163-169. PMID: 7974491.


21. Basu S, Meisert I, Eggensperger E, Krieger E, Krenn CG. Time course and attenuation of ischaemia-reperfusion induced oxidative injury by propofol in human renal transplantation. Redox Rep 2007; 12: 195-202. PMID: 17705990.


22. Fan SZ, Yu HY, Chen YL, Liu CC. Propofol concentration monitoring in plasma or whole blood by gas chromatography and high-performance liquid chromatography. Anesth Analg 1995; 81: 175-178. PMID: 7598252.


23. Bein B, Renner J, Caliebe D, Scholz J, Paris A, Fraund S, et al. Sevoflurane but not propofol preserves myocardial function during minimally invasive direct coronary artery bypass surgery. Anesth Analg 2005; 100: 610-616. PMID: 15728039.


24. Corcoran TB, Engel A, Sakamoto H, O'Callaghan-Enright S, O'Donnell A, Heffron JA, et al. The effects of propofol on lipid peroxidation and inflammatory response in elective coronary artery bypass grafting. J Cardiothorac Vasc Anesth 2004; 18: 592-604. PMID: 15578470.


25. Luo T, Xia Z. A small dose of hydrogen peroxide enhances tumor necrosis factor-alpha toxicity in inducing human vascular endothelial cell apoptosis: reversal with propofol. Anesth Analg 2006; 103: 110-116. PMID: 16790636.


Fig. 1
Effects of propofol (P) pretreatment (10 µM, 25 µM or 50 µM) on lactate dehydrogenase (LDH) release from HK-2 cells after exposure to 8 mM H2O2 for 4 hours. Propofol decreased LDH release in a concentration-dependent manner. Values are shown as mean ± SD. *P < 0.05 compared with untreated cells. †P < 0.05 compared with either 10 µM or 25 µM propofol pretreated cells.

Fig. 2
Effects of propofol (P) pretreatment (10 µM, 25 µM or 50 µM) on viable cell counts after exposure of HK-2 cells to 8 mM H2O2 for 4 hours. Propofol increased the percentage of trypan blue exclusion cells (viable cells) in a concentration-dependent manner. Values are shown as mean ± SD. *P < 0.05 compared with untreated cells. †P < 0.05 compared with either 10 µM or 25 µM propofol pretreated cells.
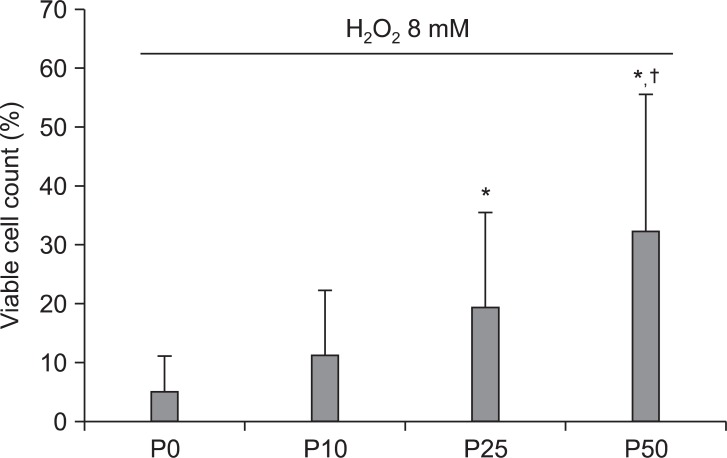
Fig. 3
Effects of propofol on H2O2-induced apoptosis and necrosis in HK-2 cells. Flow cytometric analysis of annexin V-FITC/PI double-stained cells. The cells were pretreated with propofol (10 µM, 25 µM or 50 µM) for 30 min and then incubated with 8 mM H2O2 for 4 hours. The percentages of cells were calculated using the ModFitLT V3.0 software program (mean values are given; the experiment was performed five times). In each plot, the lower left quadrant represents viable cells, the upper left quadrant indicates necrotic cells, the lower right quadrant denotes early apoptotic cells, and the upper right quadrant represents necrotic or late apoptotic cells. FITC: fluorescein isothiocyanate, PI: propidium iodide.
- TOOLS








