|
 |
|
|
Abstract
Background
This study was designed to measure in vivo effects of propofol, isoflurane and sevoflurane on apoptosis by measuring caspase-3 and tumor necrosis factor (TNF)-related apoptosis inducing ligand (TRAIL) blood level as apoptotic markers.
Methods
After obtaining ethical committee approval and informed written consents, sixty adult patients ASA I scheduled for open cholecystectomy participated in this study. They were randomally allocated into one of three equal groups to receive propofol infusion, low-flow isoflurane or sevoflurane for maintenance of anesthesia. Venous blood samples were collected preoperatively, immediately postoperative and after 24 hours to measure hemoglobin, hematocrit, creatinine, liver enzymes, serum TRAIL and caspase-3 levels.
Results
There was no significant difference in hematological markers and serum creatinine. Liver enzymes showed significant postoperative rise (P < 0.05). In Propofol group, TRAIL and caspase-3 levels were significantly elevated immediately postoperative then decreased significantly after 24-hours (P < 0.05). In Isoflurane group, immediate postoperative level of TRAIL was significantly higher than 24 hours reading and significantly lower than its level in Propofol group at the same timing meanwhile caspase-3 levels were comparable at different timings. In Sevoflurane group, TRAIL and caspase-3 levels increased significantly in both postoperative samples than preoperative level and than those of Isoflurane and Propofol groups after 24 hours concerning TRAIL (P & 0.05).
Conclusions
This study concluded that isoflurane is superior and sevoflurane is the least effective among the three anesthetics in protection against apoptosis. This study neither proved nor excluded propofol-induced apoptosis. Further studies are required during lengthy procedure and in compromised patients.
Apoptosis, or programmed cell death, is defined as distinct morphological and biochemical changes which mediated by a family of cysteine aspartases (caspases) caspase-1 to caspase-14 [1]. They are expressed as inactive zymogens that proteolytically activated following intrinsic or extrinsic stimuli [2]. Apoptosis is characterized by cytoplasmic and nuclear shrinkage, chromatin fragmentation, and breakdown of the cell into multiple spherical bodies that retain membrane integrity [3]. The tumor necrosis factor (TNF)-related apoptosis inducing ligand (TRAIL) are death cell surface receptors that induce apoptosis through activation of caspase-3 and -6 in caspase cascade [4]. Necrosis is activated mostly by extrinsic factors and is characterized by progressive loss of cytoplasmic membrane integrity, rapid influx of Na+, Ca2+, and water, resulting in cytoplasmic swelling and nuclear pyknosis [3]. The contribution of cell death as apoptosis vs. necrosis may be a determinant factor of the severity of morbidity or mortality [5].
Anesthetics may play an important role in immunomodulation through their effect on apoptosis leading to different postoperative outcome [6]. The antioxidant activity of propofol suggests its potential role to modulate apoptosis [7] while the effect of volatile anesthetics remains obscure. Sevoflurane and isoflurane might induce apoptosis in T lymphocytes via increased mitochondrial membrane permeability and caspase-3 activation [8]. On the contrary, others reported them to be protective against ischemic reperfusion injuries [9] as well as cardioprotection through their antiapoptotic effects [10].
Since scarce studies were conducted in vivo to correlate the effect of anesthesia with the clinical practice, we aimed to study the effects of propofol, isoflurane and sevoflurane on apoptosis by measuring blood level of caspase-3 and TRAIL as apoptotic markers in American Society of Anesthesiologists (ASA) physical status I patients scheduled for elective open cholecystectomy.
After obtaining Ethical Committee approval in Theodor Bilharz Research Institute and informed written consent, sixty adult patients aged between 18-60 years of either sex ASA physical status I scheduled for open cholecystectomy were enrolled in this prospective, randomized study. Patients were excluded if they refused to sign the consent form, were ASA physical status II or greater, extremes of age, obese patients (BMI > 30), pregnant, lactating or menstruating females, heavy smokers, addicts, drug abusers, or undergoing emergency operations. The patients were withdrawn from the study if they needed postoperative mechanical ventilation, perioperative corticosteroid therapy, or blood transfusion.
The enrolled patients were randomly allocated according to a computer generated randomization list to three groups of twenty patients each to receive propofol, isoflurane or sevoflurane for maintenance of anesthesia. They were premedicated with midazolam 0.05 mg/kg given intravenously half an hour before induction of anesthesia. The following monitors were attached to the patients: five leads ECG, non invasive blood pressure, SpO2, capnography, anesthetic gas analyzer, temperature and peripheral nerve stimulator (Infinity Kappa, Dräger, Lübeck, Germany). Ringer's acetate solution (500 ml) was infused as a preload then at a rate of 6-8 ml/kg/h during surgery for supplying maintenance and deficit, while blood losses were replaced by colloids (Hemohes, 6% Hes 200/0.5 B. Braun Melsungen AG, Germany) not exceeding one liter. All patients received 1 gm cefoperazone preoperatively as a standard antibiotic. Anesthesia was induced with IV fentanyl 2 µg/kg and IV propofol 1.5-2 mg/kg until loss of verbal contact. Neuromuscular blockade was achieved by IV atracurium 0.5 mg/kg followed by tracheal intubation.
In the "Propofol group": Anesthesia was maintained with intravenous propofol 10 mg/kg/h for the first 10 minutes, 8 mg/kg/h for the second 10 minutes and continued by 3-6 mg/kg/h thereafter till the end of surgical procedure. For the "Isoflurane group" and "Sevoflurane group": anesthesia was maintained to keep the end-tidal anesthetic concentrations within 0.8-1.2% for Isoflurane group or 1.5-2% for Sevoflurane group.
For all patients, fresh gas flow oxygen in air 30-40% at a rate of 1 L/min was administered using a closed system (Fabius GS, Dräger, Lübeck, Germany) and ventilation was adjusted to maintain end-tidal carbon dioxide at 35-40 mmHg. An increase in HR and/or MAP > 30% of baseline values was treated by IV fentanyl 0.5 µg/kg boluses. Atropine 0.5 mg IV increments were used to control bradycardia (< 50 beats/min) while hypotension (less than 20% of pre-anesthetic level) was managed by increasing fluid infusion rate, decreasing infusion rate of propofol/concentration of volatile anesthetic and/or using vasoactive drugs. Core temperature was measured by using an esophageal thermometer and normothermia was maintained using a forced air warming blanket and actively warmed infused solutions. Neuromuscular blockade was achieved intraoperatively with intermittent doses of IV atracurium 0.1 mg/kg when Train of Four (TOF) ratio reached 25%. At the end of surgery, reversal of neuromuscular blockade was achieved by intravenous titration of neostigmine 0.05 mg/kg and atropine 0.02 mg/kg. Mepridine 1 mg/kg was given intramuscularly every 8 hours to control postoperative pain during the first 24 hours after surgery. Monitoring of arterial blood pressure and heart rate continued postoperatively and recorded every four hours. The patient and the observers collecting data were blinded to the patient's study group allocation.
Laboratory works: Three Venous blood samples (5 ml each) were withdrawn from each patient to be divided into 2 aliquots; the 1st portion was on EDTA anticoagulant for hemoglobin (Hb) and hematocrit (Hct) measurement. The 2nd one was collected in plain tube, allowed to clot then centrifuged. The separated serum was further subdivided into two aliquots; one of them used to measure serum creatinine, alanine aminotransferases (ALT) and aspartate aminotransferases (AST). These hematological and biochemical markers were measured preoperatively and 24 hours after surgery. The second part of serum were refrigerated at -80℃ for specific assay of serum TRAIL and caspase-3 levels at the following time points; preoperative (baseline), at the end of surgery (immediate postoperative) and 24 hours postoperatively.
It was measured by using the BioSource human TRAIL kit (Catalog #KHC1632/KHC1631, California, USA) which is a solid phase sandwich Enzyme Linked-Immuno-Sorbent Assay (ELISA). A monoclonal antibody specific for human TRAIL has been coated onto the wells of the micro titer strips provided. Samples, including standards of known human TRAIL content, control specimens, and unknowns, were pipetted into these wells. During the first incubation, the Human TRAIL antigen binded to the immobilized (capture) antibody on one site. After washing, a biotinylated polyclonal antibody specific for Human TRAIL was added. During the second incubation, this antibody binded to the immobilized Human TRAIL captured during the first incubation. After removal of excess second antibody, Streptavidin-Peroxidase (enzyme) was added. This binded to the biotinylated antibody to complete the four-member sandwich. After a third incubation and washing to remove all the unbound enzyme, a substrate solution was added, which was acted upon by the bound enzyme to produce color. The intensity of this colored product is directly proportional to the concentration of Human TRAIL present in the original specimen.
Quantitative determination of serum caspase -3 was measured by using Correlate-assay, Caspase-3 Colorimetric Assay Kit, (Catalog No. 907-013). It involves conversion of a specific chromogenic substrate for caspase-3 followed by colorimetric detection of colored end-product (p-nitroaniline, p-NA) whose absorbance is directly proportional to the respective caspase-3 concentration. Using linear graph paper, the average net nominal concentration for each standard was plotted versus actual concentration of caspase-3 for the standards. The concentration of caspase-3 in samples can be determined from appropriate standard curve.
Statistical analysis: Based on our preliminary trail to detect changes in caspase-3 level and assuming that α = 0.05% and power of 80%, sample size of 20 patients per group were required to detect this difference between groups. Data were expressed as mean (standard deviation) or number (%). Comparison between numerical data in different groups was performed using ANOVA with post-hoc Bonferroni test while comparison relative to the baseline in the same group was performed using ANOVA with post-hoc Dunnet test. Comparison between categorical data was performed using Chi-square test. The data were considered significant if P value was ≤ 0.05. Statistical analysis was performed with the aid of the SPSS computer program (version 12 windows).
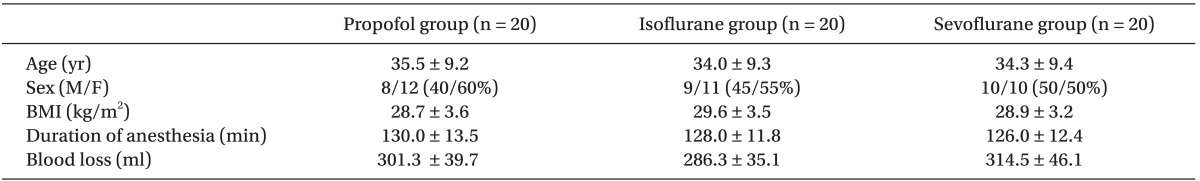
The study groups showed no significant difference concerning demographic data, duration of anesthesia and mean intraoperative blood loss (Table 1). All changes in mean intra- and post-operative blood pressure and heart rate in the three groups were within the clinical acceptable ranges and none of the patients were in need of rescue medications.
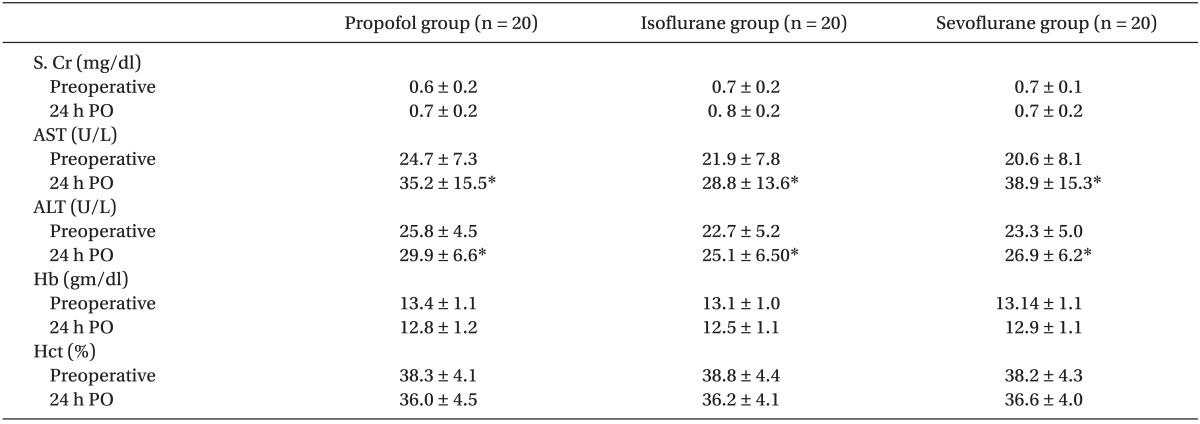
Concerning biochemical and hematological markers, serum creatinine showed a non significant rise while Hb and Hct level showed a non significant fall from preoperative level in all groups without any significant difference between groups. ALT and AST showed statistically significant rise from preoperative levels in all groups with P value < 0.05 but no significant difference between groups (Table 2).
Regarding apoptotic markers; In Propofol group, TRAIL and caspase-3 levels were significantly elevated immediately postoperative compared to preoperative level then decreased significantly after 24-hours compared to immediate postoperative reading (P < 0.05). In Isoflurane group, immediate postoperative level of TRAIL was significantly higher than 24 hours reading and significantly lower than its level in Propofol group at the same timing meanwhile caspase-3 levels were comparable at different timings within the group. In Sevoflurane group, TRAIL and caspase-3 levels increased significantly in both postoperative samples than preoperative level and than those of Isoflurane and Propofol groups after 24 hours concerning TRAIL (P < 0.05). Caspase-3 level did not show statistically significant difference between groups at different timings (Fig. 1 and 2).
This study compared the in vivo effect of isoflurane, sevoflurane and propofol on TRAIL and Caspase-3 levels as apoptotic markers. Isoflurane appeared to have the most protective effect against apoptosis as indicated by minimal postoperative changes of these markers, while sevoflurane was accompanied with postoperative increase of their levels and failed to regain the baseline values so it may have the least antiapoptotic effect. This reopens the question about the low flow sevoflurane safety and the effect of its toxic bio-products on human tissues. Also we should not ignore that caspase-3 in Propofol group returned to near preoperative level after 24 hours while TRAIL level returned to even less than baseline.
Loepke et al. [11] and Satomoto et al. [12] found similar results to this study reporting that sevoflurane caused more apoptosis and functional disability than isoflurane in neonatal brain cells of mice by using caspase-3 assay. Meanwhile Zhang et al. [13] suggested that both isoflurane and sevoflurane partially inhibited apoptosis but with no significant difference. They studied the anesthetic effects on ischemic neurons after cerebral ischemia-reperfusion in 10 rats by the expression of anti-apoptotic factor Bcl-2 mRNA and interleukin-1beta. This discrepancy may be due to their use of small sample size and different markers. Isoflurane has been reported to have neuroprotective effect against dynorphyn (Known cytotoxic compound) in cultured human neuroblastoma cells through its effect on cell survival, apoptosis, and antiapoptotic protein expression [14]. Similarly, Lin et al. [15] showed that isoflurane did not cause injury to human neuron-like cells and did not increase the expression of the activated caspase-3. In addition, isoflurane had a cardioprotective effect by inhibition of apoptosis in cardiomyocytes [16] and also inhibited apoptosis in renal medulla of rats [17].
In contrast, Yang et al. [18] suggested that isoflurane has greater potency than sevoflurane or desflurane to cause calcium release from the endoplasmic reticulum and to induce cell apoptosis. The authors used chicken β lymphocytes exposed to 2 MAC of anesthetic agents for 24 hours and determined apoptosis activity by the degree of cell damage and measuring caspase-3 level. In addition, treatment with 2% isoflurane for 6 h increased pro-apoptotic factor (Bax levels) as well as decreased anti-apoptotic factor (Bcl-2 levels), and caspase-3 and-9 in cultured cells, primary neurons, and mice [19]. These differences in results may be due to higher concentration and longer duration of anesthetic exposure in the previous two studies than that used in our study where patients were exposed to 1 MAC for about 2 hours and also in vitro studies are not enough to build a firm conclusion. Different concentrations of isoflurane treatment could have different effects as low-concentration may protect against whereas high-concentration may promote hypoxia-induced caspase-3 activation [20].
The use of sub-clinical concentration of sevoflurane proved to potentiate neuronal apoptosis in the developing mouse brain cells which was detected by immunohistochemistry of cleaved caspase-3 and electron microscopy [21]. After one hour exposure of human peripheral polymorph nuclear neutrophils to sevoflurane, oxidative stress and cellular injury were induced in a dose dependant manner by measuring the activity of caspase-3 and -7 [22]. The results of our study go in accordance with Papadima et al. [23] who reported that sevoflurane has an apoptotic effect in vivo but through measuring total lymphocyte counts and lymphocyte subpopulations (early apoptotic, late apoptotic, viable, and necrotic cells).
Concerning propofol, the present work suggests that propofol is more protective than sevoflurane though less than isoflurane, against apoptosis. However, it is neither proved nor excluded that propofol can induce apoptosis. It was suggested that propofol attenuates intestinal epithelial apoptosis in rats through reducing ceramide production, a messenger for apoptosis [24]. Propofol can also prevent dopamineinduced apoptosis after ischemia assessed by immunoblotting of caspase-3 cleavage in rabbit hearts [25]. In addition, propofol could inhibit apoptosis in astroglial cells evaluated by cytotoxicity assay and caspase-3 activation [26]. On the contrary, Siddiqui et al. [27] proposed that the anticancer effects of novel propofol conjugates on breast cancer cells significantly induced apoptosis; however, they studied propofol conjugates and not propofol itself. In another work conducted by Tsuchiya et al. [28], they found that propofol treatment could activate caspase-3, -6, -8 and -9. By using immunofluorescence and western-blot analyses. Engelhard et al. [29] showed that sevoflurane and propofol had equal effect on the expression of apoptosis-regulating proteins (Bax, Bcl-2). This dissimilarity in results may be due to different markers used and the later study was conducted after ischemia and reperfusion which cause apoptosis via different mechanism.
Preoperative levels of apoptotic markers (caspase-3 and TRAIL levels) in the present study were comparable between the three groups starting from almost the same baseline. Estimating the serum level of these markers in vivo is an easy, non-invasive and reliable method especially that the two-markers were changed in a parallel pattern and are activated in the early stages of apoptosis [30]. Disadvantage of the present study is that it did not identify the organ subjected to apoptosis or protected, in contrast to in vitro or animal studies that examine cells from each organ. ASA I patients were only included in this study to minimize any existing effects of systemic diseases on apoptosis. Pediatric patients were excluded as they have developing organs, which may affect the results [12]. Perioperative hemodynamic data and blood loss did not manifest wide changes or subjected to extensive surgical intervention. This study evaluated the perioperative creatinine level to rule out any changes in the kidney functions. On the other hand postoperative levels of liver enzymes were elevated than preoperative levels within each group as any procedure near the liver can elevate liver enzymes, but they were comparable between groups.
This study concludes that isoflurane is the superior and sevoflurane is the least effective among the three anesthetics in protecting against apoptosis in human cells. However, it is neither proved nor excluded that propofol induce apoptosis. Further studies are recommended to evaluate the usage of isoflurane for anesthesia maintenance in lengthy procedures. The link of the apoptotic markers level to the ASA II, III status and compromised patients are also recommended. Measuring the pre anesthetic serial levels of apoptotic markers days before surgery is required to estimate their baseline average level without immediate preoperative stress effect. Also increasing the duration of follow up after surgical intervention with more frequent measuring is needed.
Acknowledgments
The authors gratefully acknowledge Dr. Mohamed A. Maher, for his valuable assistance in this work and Dr. Tarek M. Diab, for statistical consultation, both of Theodor Bilharz Research Institute, Giza, Egypt.
References
1. Thornberry NA, Lazebnik Y. Caspases: enemies within. Science 1998; 281: 1312-1316. PMID: 9721091.


2. Padanilam BJ. Cell death induced by acute renal injury: a perspective on the contributions of apoptosis and necrosis. Am J Physiol Renal Physiol 2003; 284: F608-F627. PMID: 12620919.


3. Majno G, Joris I. Apoptosis, oncosis, and necrosis. An overview of cell death. Am J Pathol 1995; 146: 3-15. PMID: 7856735.


4. Crowder CM. Cell biology. Ceramides-friend or foe in hypoxia? Science 2009; 324: 343-344. PMID: 19372418.



5. Bhatia M. Apoptosis versus necrosis in acute pancreatitis. Am J Physiol Gastrointest Liver Physiol 2004; 286: G189-G196. PMID: 14715516.


6. Pape M, Engelhard K, Eberspächer E, Hollweck R, Kellermann K, Zintner S, et al. The long-term effect of sevoflurane on neuronal cell damage and expression of apoptotic factors after cerebral ischemia and reperfusion in rats. Anesth Analg 2006; 103: 173-179. PMID: 16790648.


7. Mellon RD, Simone AF, Rappaport BA. Use of anesthetic agents in neonates and young children. Anesth Analg 2007; 104: 509-520. PMID: 17312200.


8. Loop T, Dovi-Akue D, Frick M, Roesslein M, Egger L, Humar M, et al. Volatile anesthetics induce caspase-dependent, mitochondria-mediated apoptosis in human T lymphocytes in vitro. Anesthesiology 2005; 102: 1147-1157. PMID: 15915027.


9. Brée B, Gourdin M, De Kock M. Anesthesia and cerebral apoptosis. Acta Anaesthesiol Belg 2008; 59: 127-137. PMID: 19051443.

10. Frässdorf J, Borowski A, Ebel D, Feindt P, Hermes M, Meemann T, et al. Impact of preconditioning protocol on anesthetic-induced cardioprotection in patients having coronary artery bypass surgery. J Thorac Cardiovasc Surg 2009; 137: 1436-1442. PMID: 19464461.


11. Loepke AW, Istaphanous GK, McAuliffe JJ 3rd, Miles L, Hughes EA, McCann JC, et al. The effects of neonatal isoflurane exposure in mice on brain cell viability, adult behavior, learning, and memory. Anesth Analg 2009; 108: 90-104. PMID: 19095836.


12. Satomoto M, Satoh Y, Terui K, Miyao H, Takishima K, Ito M, et al. Neonatal exposure to sevoflurane induces abnormal social behaviors and deficits in fear conditioning in mice. Anesthesiology 2009; 110: 628-637. PMID: 19212262.


13. Zhang SD, Zhai J, Zhang H, Wan H, Li DZ. Protective effect of isoflurane and sevoflurane on ischemic neurons and expression of Bcl-2 and ICE genes in rat brain. Biomed Environ Sci 2006; 19: 143-146. PMID: 16827187.

14. Wu GJ, Chen WF, Sung CS, Jean YH, Hung CH, Chen FA, et al. Isoflurane attenuates dynorphin-induced cytotoxicity and down regulation of Bcl-2 expression in differentiated neuroblastoma SH-SY5Y cells. Acta Anaesthesiol Scand 2009; 53: 55-60. PMID: 19032555.


15. Lin D, Feng C, Cao M, Zuo Z. Volatile anesthetics may not induce significant toxicity to human neuron-like cells. Anesth Analg 2011; 112: 1194-1198. PMID: 20966438.


16. Hu ZY, Luo NF, Liu J. The protective effects of emulsified isoflurane on myocardial ischemia and reperfusion injury in rats. Can J Anaesth 2009; 56: 115-125. PMID: 19247759.


17. Aravindan N, Cata JP, Hoffman L, Dougherty PM, Riedel BJ, Price KJ, et al. Effects of isoflurane, pentobarbital, and urethane on apoptosis and apoptotic signal transduction in rat kidney. Acta Anaesthesiol Scand 2006; 50: 1229-1237. PMID: 16978161.


18. Yang H, Liang G, Hawkins BJ, Madesh M, Pierwola A, Wei H. Inhalational anesthetics induce cell damage by disruption of intracellular calcium homeostasis with different potencies. Anesthesiology 2008; 109: 243-250. PMID: 18648233.



19. Zhang Y, Dong Y, Wu X, Lu Y, Xu Z, Knapp A, et al. The mitochondrial pathway of anesthetic isoflurane-induced apoptosis. J Biol Chem 2010; 285: 4025-4037. PMID: 20007710.


20. Pan C, Xu Z, Dong Y, Zhang Y, Zhang J, McAuliffe S, et al. The potential dual effects of anesthetic isoflurane on hypoxia-induced caspase-3 activation and increases in â-site amyloid precursor protein-cleaving enzyme levels. Anesth Analg 2011; 113: 145-152. PMID: 21519046.



21. Zhang X, Xue Z, Sun A. Subclinical concentration of sevoflurane potentiates neuronal apoptosis in the developing C57BL/6 mouse brain. Neurosci Lett 2008; 447: 109-114. PMID: 18852026.


22. Wong CH, Liu TZ, Chye SM, Lu FJ, Liu YC, Lin ZC, et al. Sevoflurane-induced oxidative stress and cellular injury in human peripheral polymorphonuclear neutrophils. Food Chem Toxicol 2006; 44: 1399-1407. PMID: 16678324.


23. Papadima A, Boutsikou M, Lagoudianakis EE, Kataki A, Konstadoulakis M, Georgiou L, et al. Lymphocyte apoptosis after major abdominal surgery is not influenced by anesthetic technique: a comparative study of general anesthesia versus combined general and epidural analgesia. J Clin Anesth 2009; 21: 414-421. PMID: 19833274.


24. Liu KX, Chen SQ, Huang WQ, Li YS, Irwin MG, Xia Z. Propofol pretreatment reduces ceramide production and attenuates intestinal mucosal apoptosis induced by intestinal ischemia/reperfusion in rats. Anesth Analg 2008; 107: 1884-1891. PMID: 19020134.


25. Roy N, Friehs I, Cowan DB, Zurakowski D, McGowan FX, del Nido PJ. Dopamine induces postischemic cardiomyocyte apoptosis in vivo: an effect ameliorated by propofol. Ann Thorac Surg 2006; 82: 2192-2199. PMID: 17126134.


26. Acquaviva R, Campisi A, Raciti G, Avola R, Barcellona ML, Vanella L, et al. Propofol inhibits caspase-3 in astroglial cells: role of heme oxygenase-1. Curr Neurovasc Res 2005; 2: 141-148. PMID: 16181106.


27. Siddiqui RA, Zerouga M, Wu M, Castillo A, Harvey K, Zaloga GP, et al. Anticancer properties of propofol-docosahexaenoate and propofol-eicosapentaenoate on breast cancer cells. Breast Cancer Res 2005; 7: R645-R654. PMID: 16168109.




28. Tsuchiya M, Asada A, Arita K, Utsumi T, Yoshida T, Sato EF, et al. Induction and mechanism of apoptotic cell death by propofol in HL-60 cells. Acta Anaesthesiol Scand 2002; 46: 1068-1074. PMID: 12366500.


29. Engelhard K, Werner C, Eberspächer E, Pape M, Blobner M, Hutzler P, et al. Sevoflurane and propofol influence the expression of apoptosis-regulating proteins after cerebral ischaemia and reperfusion in rats. Eur J Anaesthesiol 2004; 21: 530-537. PMID: 15318464.


30. Belizário JE, Alves J, Occhiucci JM, Garay-Malpartida M, Sesso A. A mechanistic view of mitochondrial death decision pores. Braz J Med Biol Res 2007; 40: 1011-1024. PMID: 17665037.



Fig. 1
Mean values of TRAIL (pg/ml). *P < 0.05 vs. preoperative values within the same group. †P < 0.05 vs. immediate postoperative values within the same group. ‡P < 0.05 vs. Popofol group at the same timing. §P < 0.05 vs. Isoflurane group at the same timing.

Fig. 2
Mean values of caspase-3 (U/ml). *P < 0.05 vs. preoperative values within the same group. †P < 0.05 vs. immediate postoperative values within the same group.
- TOOLS