Perioperative effects of various anesthetic adjuvants with TIVA guided by bispectral index
Article information
Abstract
Background
This prospective, randomized, double blinded, controlled study was designed to compare effects of intravenous co-administration of clonidine, magnesium, or ketamine on anesthetic consumption, intraoperative hemodynamics, postoperative analgesia and recovery indices during Bispectral Index (BIS) guided total intravenous anesthesia (TIVA).
Methods
After ethical committee approval and written informed consent, 120 adult patients ASA I and II scheduled for open cholecystectomy were randomly assigned to one of 4 equal groups. Group CL received clonidine 3 µg/kg and maintained by 2 µg/kg/h. Group MG received magnesium sulphate 50 mg/kg and maintained by 8 mg/kg/h. Group KET received racemic ketamine 0.4 mg/kg and maintained by 0.2 mg/kg/h. Control group (CT) received the same volume of isotonic saline. Anesthesia was induced and maintained by fentanyl, propofol and rocuronium. Propofol infusion was adjusted to keep the BIS value between 45-55. Intraoperative hemodynamics, induction time, anesthetic consumption, recovery indices, and PACU discharge were recorded.
Results
Induction time, propofol requirements for induction and maintenance of anesthesia, intraoperative fentanyl and hemodynamic values were significantly lower with Groups CL and MG compared to Groups KET and CT (P < 0.05). Patients in Group MG showed significantly lower muscle relaxant consumption, delayed recovery and PACU discharge than other groups (P < 0.05). First, analgesic requirement was significantly longer and total postoperative analgesic consumption was significantly lower in the adjuvant groups versus Group CT (P < 0.05).
Conclusions
Clonidine, magnesium, and ketamine can be useful adjuvant agents to BIS-guided TIVA. Pharmacokinetic studies of such drug combinations were recommended to investigate their interaction.
Introduction
Clonidine is an alpha2-adrenergic agonist with sedative and analgesic properties that reduces the requirement for propofol [1]. The extent and nature of the reduction is not accurately monitored by hemodynamic end-points, since they do not reflect an adequate level of anesthesia [2]. Magnesium sulphate (MgSO4) has analgesic and sedative properties secondary to the blockade of N-methyl-D-aspartate (NMDA) receptors and reduction of catecholamine release [3]. It has been suggested as a good adjuvant to TIVA [4], although this has not been verified [5]. In a subanesthetic dose, ketamine possesses analgesic properties through NMDA receptors antagonism with minimal side effects [6]. We hypothesized that adding these drugs to a propofol-fentanyl combination guided by the bispectral Index (BIS), might optimize TIVA consumption and minimize intraoperative hemodynamic changes, with a faster recovery. To our knowledge, comparison between the 3 adjuvants with BIS-guided TIVA has not been studied previously. Thus, this prospective, randomized, double blinded, placebo controlled study was designed to compare the effects of the co-administration of IV clonidine, magnesium sulphate, or racemic ketamine with BIS-guided TIVA, primarily on propofol, fentanyl, and rocuronium consumption, and secondarily on the intraoperative hemodynamics and recovery indices.
Materials and Methods
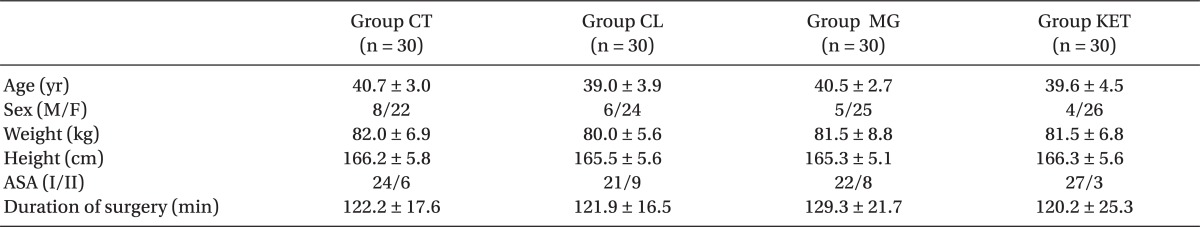
After obtaining ethical committee approval and written informed consent, 120 adult male and female patients between ages 18-60 years old American Society of Anesthesiologists (ASA) physical status I and II scheduled for open cholecystectomy under general anesthesia were enrolled in this study. Patients were randomly allocated to one of 4 groups of 30 patients each according to a computer generated randomization list: Group CL (clonidine group), Group Mg (magnesium group), Group KET (ketamine group), and Group CT (control group). Patients with the following are excluded from the study: hepatic, renal, respiratory, cardiac or central nervous system (CNS) dysfunction, seizure disorders, obesity (BMI > 30), pregnancy, prior treatment with calcium channel blockers and known allergy to study drugs. On arrival to the operating room, patients were infused with lactated Ringer's solution at a rate 8-12 ml/kg/h, which was continued throughout the operation. The following monitors were attached to patients: 5-lead ECG, noninvasive blood pressure, pulse oximetry, PETCO2 and neuromuscular monitoring (Infinity Kappa, Dräger, Lübeck, Germany). BIS module (Infinity® BISx™ SmartPod®, smoothing rate: 15 or 30 seconds, software revision: VF5) was attached to the monitor for detection of consciousness level. Disposable BIS electrodes (BIS quatro, Aspect Medical Systems, USA) were placed on the forehead after proper preparation of the skin. Nasopharyngeal temperature was monitored and a normal temperature was maintained. All basic readings were recorded before the start of anesthesia.
A loading dose of anesthetic adjuvants was given IV as premedication in 100 ml saline over 15 min in the following regimen: Group CL received 3 µg/kg clonidine hydrochloride, Group MG received 50 mg/kg MgSO4, and Group KET received 0.4 mg/kg racemic ketamine hydrochloride, while Group CT received the same volume as isotonic saline. Immediately afterward, anesthesia was induced by IV fentanyl 1.5 µg/kg and propofol in a dose of 10 mg every 5 seconds until the BIS level dropped below 60. The time from the start of propofol injection until BIS level dropped to 60 was considered as the induction time. Maintenance of anesthesia was initiated by propofol infusion at a rate 200 µg/kg/min immediately after induction and then titrated to maintain the BIS value between 45 and 55. This rate was changed by increments of 20 µg/kg/min if the BIS value went out of the targeted range for more than 15 seconds. Anesthetic adjuvants were infused at a preset infusion rate prepared by an anesthesiologist not involved in the study in 50 ml increments through a syringe pump as follows: 2 µg/kg/h clonidine HCl in Group CL, 8 mg/kg/h MgSO4 in Group MG, 0.2 mg/kg/h racemic ketamine HCl in Group KET or isotonic saline in the Group CT. The guidance for the doses in the study were provided by previous studies, [7,8] while a subanesthetic dose of ketamine was selected to achieve analgesia with minimal psychotomimetic side effects [6].
The ulnar nerve was stimulated supramaximally with repetitive train of four (TOF) stimuli. After calibration, rocuronium 0.6 mg/kg was administered IV until TOF ratio reaches 0-5% then trachea was intubated and lungs were ventilated with 50% oxygen in air. The following neuromuscular parameters were recorded: onset time, duration of action and recovery index. If BIS level remained within the targeted range while mean arterial pressure (MAP) or heart rate (HR) exceeded 20% of baseline values, fentanyl boluses of 0.5 µg/kg were given. Hemodynamics were recorded preoperatively, after premedication infusion, induction of anesthesia, intubation, before and after skin incision then every 10 minutes interval till end of the procedure. If MAP dropped below the acceptable clinical range (i.e. < 60 mmHg), the patient was treated with IV ephedrine 6 mg increments, while HR below 40 beats/min was managed by IV atropine 10 µg/kg. Once the first twitch in the TOF (T1) recovered to 25% of its baseline height, muscle relaxation was maintained by incremental doses of rocuronium 0.12 mg/kg.
After skin closure, all infusion drugs were stopped and antagonism of residual neuromuscular block was carried out by 35 µg/kg neostigmine together with 20 µg/kg atropine when T4/T1 ratio reached 75% or higher followed by tracheal extubation. The time from stoppage of propofol infusion until BIS level raised to 80 was considered as the recovery time. The time from cessation of anesthetics to tracheal extubation, response to verbal commands and orientation in time were also recorded. Patients were then transferred to the post anesthesia care unit (PACU), where they were monitored. Sedation score was assessed on reaching PACU using the 4 categorical scale: 0 = alert and aware; 1 = drowsy, not sleeping; 2 = asleep, arousable by verbal contact and 3 = asleep not arousable by verbal contact. They were discharged from PACU only when they reached an Aldrete score of 10 (ie, when they were able to move all extremities in response to a request, able to breathe deeply and cough freely, stable systemic blood pressure [± 20% of pre-anesthetic level], fully awake and had oxygen saturation > 92% while breathing room air). Induction dose of propofol (mg/kg), the amount of propofol infused after the bolus (mg/kg/h), total amount of fentanyl (µg/kg/h) and total amount of rocuronium (mg/kg/h) after induction were also calculated by dividing the total dose of each drug by the patient's body weight and the anesthesia time. Postoperative analgesic consumption over 24 hours was calculated according to Visual analog scale (VAS) score for pain assessment in which 0 = no pain to 10 = maximal pain. When VAS exceeded 3, rescue analgesia in the form of meperidine 1 mg/kg was given IV. Any postoperative side effects, like nausea, vomiting, bradycardia, hypotension, excessive sedation, hallucination, nightmares, or diplopia was recorded. Patients who were experiencing nausea or vomiting received ondansetron 4 mg IV as a rescue anti-emetic.
Statistical analysis
Based on a previous study [9], sample size was calculated according to propofol consumption as a primary goal (standardized Δ was 0.857 between groups). Assuming α = 0.05 and 1-β (power level) = 90%, 30 patients per group were required to detect this difference. Results are expressed as means ± standard deviation (SD) or numbers. Comparison between numerical data in different groups was performed using ANOVA with post-hoc Bonferroni test, while comparison relative to the baseline in the same group was performed using ANOVA with post-hoc Dunnet test. Comparison between categorical data was performed using Chi-square test. The data were considered significant if P value was < 0.05. Statistical analysis was performed with the aid of the SPSS computer program (version 12 windows).
Results
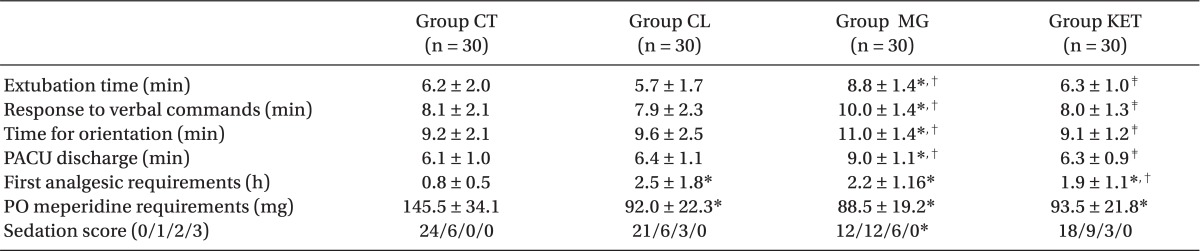
Patients in the 4 groups were comparable with respect to age, sex, body weight, height, ASA status and duration of surgery (Table 1). The induction time was significantly shorter in Group CL followed by Group MG when compared with Groups KET and CT (P < 0.05) (Table 2). The recovery time was significantly longer in Group MG and significantly shorter in Group CL compared to other groups (P < 0.05) (Table 2). Requirements for induction and maintenance of propofol and intraoperative fentanyl were significantly lower in Groups CL and MG compared to Groups KET and CT, with further reduction in Group CL versus Group MG. Rocuronium need for intra-operative relaxation was significantly lower in Group MG compared to other groups (P < 0.05) (Table 2). Concerning neuromuscular monitoring, Group MG showed significant shorter onset with significant longer duration and recovery index compared with other groups (P < 0.05), while there was no significant difference between the remaining groups (Table 2).

Induction (BIS < 60), Recovery (BIS > 80) Times, Anesthetic Requirements and Neuromuscular Monitoring Data
Extubation time, response to verbal commands, time for orientation and PACU discharge were significantly longer in Group MG versus other groups (P < 0.05) (Table 3). The time to first analgesic requirement was significantly longer and postoperative 24 hours meperidine consumption was significantly lower in the 3 adjuvant groups versus the Group CT (P < 0.05) (Table 3). Patients in Group MG were significantly sedated than those in Group CT with no significant difference between other groups (Table 3).
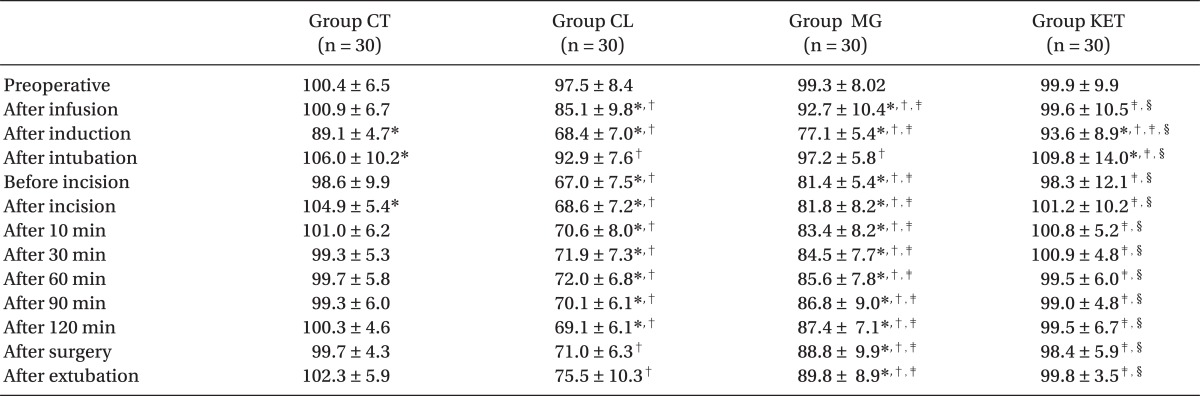
MAP and HR values were significantly lower after infusion of premedication drug in the Groups CL and MG and after induction in all groups compared with preoperative readings (P < 0.05). After intubation, both hemodynamic parameters showed significant rise in the Groups CT and KET (P < 0.05), and non-significant rise in the Groups CL and MG. compared with preoperative value. Otherwise, they were significantly reduced throughout the procedure in the Groups CL and MG compared with preoperative level and also with the other two groups, Group CL being the lowest (P < 0.05). After surgery, HR showed non-significant reduction in the Groups CL and MG and significant rise in the Group CT (P < 0.05) (Tables 4 and 5).
Regarding side effects; one patient in the Group CT experienced nausea and vomiting who was treated by IV ondansetron 4 mg. Another patient in the Group KET had hallucinations on emergence. A third patient showed intraoperative hypotension in the Group CL and was treated once with 6 mg IV ephedrine. No other side effect was recorded.
Discussion
This study showed that co-administration of clonidine or magnesium as adjuvants to TIVA guided by BIS reduced anesthetic consumption, lowered hemodynamic values and enhanced postoperative analgesia, however the recovery indices were prolonged by magnesium. These results agree with previous studies [7,9] as well as with Singh and Arora [10] concerning clonidine.
The induction dose of propofol is reduced by using clonidine. Although we did not perform a pharmacokinetic study, the most plausible explanation is that clonidine, like other alpha2-agonists, reduces cardiac output and is likely to reduce hepatic blood flow, which affects in turn the volume of distribution of propofol [11].
Clonidine infusion reduced intraoperative propofol consumption in the current study by 33%. Fehr et al. [2] and Imai et al. [12] studies reported propofol saving by using clonidine of about 18% and 42%, respectively. Less propofol reduction, observed by Fehr et al. [2] in comparison to our study, may be referred for their use of clonidine once over 10 minutes after induction of anesthesia without using it, neither as a premedication nor for maintenance throughout the procedure. The greater propofol reduction in Imai et al. [12] may be explained by their adjustment of the propofol dose according to arterial blood pressure and heart rate without any monitoring of the depth of anesthesia.
Similar to the current study, clonidine reduced intraoperative fentanyl doses as reported by Altan et al. [7] and Ray et al. [9] and the postoperative analgesic requirement as reported by Morris et al. [13]. Clonidine is an alpha-2 receptor agonist that activates inhibitory pathways in CNS, causing anxiolysis and analgesia, thus reducing the anesthetic and opioid requirements [14]. In contrast, Fehr et al. [2] found that clonidine did not have any additional effect in reducing the intra-operative requirement for opioid. They supposed that epidural administration of clonidine is more effective than systemic route against the intense intra-operative pain because the primary clonidine analgesic site proposed to be the spinal dorsal horn. However, the continuous IV pre- and intra-operative clonidine infusion regimen in this study significantly enhanced analgesia.
Clonidine did not show any significant effect on rocuronium used for muscle relaxation concerning its consumption, dose, onset, duration or recovery. Clonidine does not increase acetylcholine release from the neuromuscular junction nor does it enhance neuromuscular contraction in vitro [15]. Clonidine in this study showed lower hemodynamic values during anesthesia and surgery, which coincide with other studies [7,9]. Clonidine reduces catecholamine release and sympathetic outflow which effectively decreases blood pressure, heart rate, peripheral resistance, and renal vascular resistance [14]. The sedative action of clonidine could delay discharge from the recovery room, but this was not observed in this study. Morris et al. [13] and Fehr et al. [2] found that clonidine did not prolong recovery times. Possible explanations include a limited sedative effect of clonidine, less propofol and opioid requirements.
This study showed that IV magnesium sulphate infusion significantly reduced intraoperative propofol, fentanyl and rocuronium requirements, and postoperative analgesic consumption like the results of Seyhan et al. [16]. Another study using epidural analgesia found that MgSO4 did not reduce the severity of pain after surgery [5]. It is possible that the superior analgesic efficacy of epidural might have masked the analgesia-potentiating effect of MgSO4 in their study. Magnesium sulphate infusion has been reported to reduce analgesic and muscle relaxant requirements, but has no effect on propofol requirements in patients undergoing gynecological surgery [4]. The discrepancy in results may be due to the difference in anesthetic dose, Mg dose, analgesic drug, and its route of administration, patient category and surgical operations of different degrees of pain.
Our results confirm clinical studies demonstrating lower neuromuscular blocker requirements with magnesium use [16,17]. Moreover, magnesium infusion with TIVA hastened onset time, prolonged duration of action, and with a recovery index of rocuronium. Kussman et al. [18] found the same concerning rocuronium duration of action without affecting speed of onset. This may be due to their use of single dose of MgSO4 without continuous maintenance of infusion. Fuchs-Buder et al. [19] demonstrated that prior administration of MgSO4 prolonged clinical duration of intermediate acting nondepolarizing neuromuscular blocking agents due to inhibition of acetylcholine release at motor nerve terminals.
Similar to our results, Ryu et al. [4] showed lower trends of hemodynamic parameters with attenuation of stress response to intubation and surgery in the magnesium group. The effects of Mg might be explained by the vasodilatation secondary to calcium channel blockade, or by NMDA receptors antagonism in the CNS with reduction of catecholamine release, decreased peripheral nociceptor sensitization, and the stress response to surgery [3]. Kussman et al. [18] found no significant difference in heart rate between magnesium and control groups immediately after induction of anesthesia. The small samples used in their study increased the likelihood of encountering such a difference randomly. Tramer et al. [17] showed similar results to our study that recovery indices and PACU discharge time were prolonged in the Group MG, but not to the extent to be a clinical problem.
In this study, clonidine was more effective in reducing intraoperative anesthetic consumption and attenuating hemodynamic stress response in comparison to magnesium group.
Our results showed that coadministration of racemic ketamine with TIVA prolonged the time to first analgesic request and reduced postoperative analgesic requirements by 36%. Menigaux et al. [20] and Adam et al. [21] similarly found that a small dose of ketamine saved postoperative opioid consumption by about 50% and 35% respectively with delayed first analgesic request in the former study. The greater analgesic reduction in the Menigaux et al. [20] may be due to different types of surgery (knee arthroscopy), which is considered less painful than the abdominal surgery in this study. Similarly, Suzuki et al. [22] stated that small-dose ketamine reduced postoperative morphine requirements and pain by 40% and 35% respectively. Laskowski et al. [23] concluded that an intravenous ketamine is an effective adjunct for postoperative analgesia particularly in painful procedures whatever the type of opioid administered, timing of ketamine administration, and ketamine dose. Menigaux et al. [20] and Adam et al. [21] showed that ketamine did not affect pain scores at rest, probably because all their patients used the PCA correctly to obtain an adequate and comparable analgesia. Activation of NMDA receptors in the spinal cord has been shown to develop and maintain central neuron sensitization during the post-surgical period. Thus, NMDA receptor antagonist such as ketamine may explain the long-lasting postoperative analgesia [24].
In this study, the intraoperative anesthetic consumption was comparable between ketamine and control groups that refer to the effect of ketamine to increase BIS readings in the anesthetized patients [25,26]. In contrast, Vereecke et al. [27] concluded that ketamine administration alone or with rocuronium could not affect BIS readings significantly so that the clinical setting should be considered during the interpretation of all indices. In addition, Faraoni et al. [28] found that BIS values did not increase with ketamine in the absence of surgical stimulation. They removed the surgical pain effect on the EEG cortical measurements, which was reported to increase the BIS readings [29]. Jaksch et al. [30] also demonstrated that the addition of ketamine to TIVA did not have any enhancing effect on intraoperative anesthetic consumption or the postoperative analgesia. They found that hemodynamic data and pain scores in the ketamine group were comparable with the control groups, possibly because of the clinically routine perioperative opioid analgesia and small sample size used.
Hemodynamic parameters were almost similar between ketamine and control groups with no protection during stress. No side effect was recorded in the Group KET except in one patient who experienced hallucinations on emergence. At the anesthetic doses of ketamine (1-3 mg/kg), more than one third of patients may have acute psychosis-like symptoms. Analgesic doses of ketamine 150-500 µg/kg produce antinociception, but may be associated with cognitive, perceptual, mood disturbances, and psychotomimetic side effects [6]. Suzuki et al. [22] found that smaller doses of ketamine did not increase the incidence of these side effects when compared to the control group.
Co-administration of clonidine or magnesium with TIVA reduced anesthetic consumption, lower hemodynamic values and better postoperative analgesia with the upper hand to clonidine. The recovery indices were prolonged by magnesium. Ketamine enhanced postoperative analgesia. Thus, these agents in the dose regimen studied appear to be useful adjuvants to BIS-guided TIVA. Pharmacokinetic studies should be conducted to investigate theinteraction of these drug combinations.
Acknowledgments
The authors gratefully appreciate Dr Tarek Diab, Assistant Professor in the Theodor Bilharz Research Institute, for his statistical consultation.
Notes
Abstract of this article was represented as poster discussion at International Anesthesia Research Society (IARS) Annual meeting, May 21-24 (2011), Vancouver, Canada.