|
 |
|
|
Abstract
Background
Remifentanil has been shown to be effective at treating potentially adverse hemodynamic responses to tracheal intubation even at low doses (< 1 µg/kg/min), which needs to be evaluated in patients with diverse cardiovascular conditions.
Methods
A low-dose regimen of remifentanil (continuous infusion of 0.1 µg/kg/min, preceded by 0.5 µg/kg bolus) was given before induction with bolus propofol and rocuronium, and heart rate as well as systolic, diastolic, and mean arterial pressures were measured at 1 min intervals from before induction to 5 min after tracheal intubation in normotensive patients, untreated hypertensive patients, and patients with known hypertension.
Results
The low-dose regimen of remifentanil resulted in parallel hemodynamic responses in all three groups, and was effective at limiting hemodynamic responses to tracheal intubation without excessive cardiovascular depression. Hemodynamic responses in our study showed a similar pattern to that reported in previous investigations, except for elevations in heart rate and arterial pressures over the baseline values immediately after intubation.
Conclusions
We suggest that the low-dose regimen of remifentanil in our study could be routinely used to modify hemodynamic responses to tracheal intubation in patients with diverse hemodynamic characteristics. However, the development of supplementary regimens is still needed to control the brief, but exaggerated responses to tracheal intubation, especially in untreated hypertensive patients.
Tracheal intubation can cause augmented sympathetic responses, resulting in tachycardia and/or hypertension, which are potentially deleterious to susceptible patients [1]. Patients with hypertension, whether treated or not, are prone to much greater swings of arterial pressure during induction and intubation, and are at greater risk of adverse events, including myocardial ischemia [2]. Various agents were shown to be effective at treating adverse hemodynamic responses during induction and tracheal intubation, such as anesthetics [3], analgesics [4,5], vasodilators [6], and sympathetic blocking agents [7]. Remifentanil is a recently developed opioid agent with potent analgesic effects characterized by rapid onset and offset, and is seemingly ideal for noxious but brief stimulation of tracheal intubation [8-10]. Hypertensive patients may not only have an exaggerated cardiovascular response to a noxious stimulus such as tracheal intubation, but also are at greater risk for developing hypotension after induction with potent anesthetics [2]. Therefore, a minimal but effective dosage of remifentanil needs to be determined. Low-dose remifentanil, being less than the manufacturer-recommended induction dose (1 µg/kg IV bolus followed by continuous infusion of 0.5-1 µg/kg/min), was previously demonstrated to be equally effective at treating hemodynamic responses to tracheal intubation, compared with higher doses of remifentanil, and alfentanil in normotensive and hypertensive patients, respectively [11,12]. It has not yet been clarified, however, whether low-dose remifentanil has an equal effect on hemodynamic responses to tracheal intubation between normotensive and hypertensive patients, especially in Koreans, who were previously shown to have reduced sensitivity to remifentanil, likely resulting from ethnic differences [13,14].
The aim of this study was to evaluate the effects of low-dose remifentanil on hemodynamic responses to tracheal intubation in patients with untreated and treated hypertension, as well as in normotensive patients.
After obtaining approval from institutional review board of our hospital, and informed consent from all patients to use the hemodynamic data collected, 100 adult patients with American Society of Anesthesiologists physical status I-II, who were scheduled for elective spine surgery were recruited for this anonymous study. Patients with no history of antihypertensive medication and with a baseline hemodynamic of systolic arterial pressure (SAP) < 140 mmHg and diastolic arterial pressure (DAP) < 90 mmHg were assigned to the normotensive group (Group N), while those with a baseline hemodynamic of SAP > 140 mmHg or DAP > 90 mmHg were assigned to the untreated hypertensive group (Group UH). The baseline hemodynamic was an averaged value of duplicate hemodynamic measurements performed before induction of anesthesia. Patients with a history of antihypertensive medication(s) for > 6 months were assigned to the known hypertension group (Group KH). Any patient with anticipated difficulty with airway maintenance and/or intubation, concurrent diagnosis of congestive heart failure, renal failure, insulin-dependent diabetes mellitus or symptomatic arrhythmia, was excluded.
Patients in Group KH were allowed to take their morning dose of antihypertensive medication(s) with sips of water on the day of surgery. Before leaving the ward, patients were provided with 0.05 mg/kg of midazolam intramuscularly as premedication. When patients arrived in the operating theater, glycopyrrolate 0.2 mg was given intravenously after confirming the patency of peripheral intravenous (IV) access. Standard monitoring included ECG, SpO2, non-invasive blood pressure, and end-tidal CO2 (ETCO2).
Remifentanil infusion of 0.1 µg/kg/min was initiated before induction of anesthesia, and maintained throughout the operation. Infusion of remifentanil was preceded by a bolus dose of remifentanil 0.5 µg/kg via a peripheral IV line. Anesthesia was induced with IV propofol 1 mg/kg over 30 sec, with an additional 10 mg administered every 10 sec until loss of verbal response. IV Lidocaine 30 mg was added to prevent injection pain with propofol. After loss of consciousness, rocuronium 0.6 mg/kg was given for neuromuscular relaxation while manual ventilation with 100% O2 (≥ 3 L/min) via a mask was maintained. Tracheal intubation was performed 3 min after induction of anesthesia with direct laryngoscopy for thoracic or lumbar surgery, or with a lightwand for cervical surgery. Then, mechanical ventilation was adjusted to have an ETCO2 of 35-40 mmHg, and 1.5 vol% sevoflurane with 60% O2 in air (2-3 L/min) was used to maintain anesthesia. Hemodynamic measurements including heart rate (HR) and systolic, diastolic, and mean arterial pressures (SAP, DAP, and MAP) were performed at 1 min intervals from before induction of anesthesia (baseline) to 5 min after tracheal intubation.
If SAP and/or HR increased by > 30% of baseline for > 60 sec (hypertension and/or tachycardia), the following treatments were performed as rescue therapy: an additional bolus dose of IV remifentanil 0.5 µg/kg was given initially, and if hypertension and/or tachycardia persisted thereafter, sevoflurane 1.5 vol% was added. If SAP decreased by > 30% of baseline for > 60 sec (hypotension), IV ephedrine was given in 2 mg increments per min until SAP improved within 30% of baseline. If HR was reduced by > 30% of baseline for > 60 sec (bradycardia), IV atropine 0.5 mg was given.
Based on previous investigations, power analysis suggested that > 30 patients per group would enable detection of a > 15 mmHg difference in MAP between groups with a chance of 80% (α = 0.05, β = 0.2) [11,12]. Data are presented as mean ± SD, median (interquartile range) or number of patients. Statistical analyses were performed using SPSS 15.0 for Windows (SPSS Inc, Chicago, IL, USA). Categorical data were examined using a chi-square test to compare groups. Continuous variables, such as demographic data, intubation-related data, and drug dosage, were tested with one-way ANOVA or a Kruskal-Wallis test, according to the normality test. Statistical analyses of hemodynamic data were performed as follows: one-way ANOVA was used for cross-sectional comparison between groups, and repeated measures ANOVA was used for longitudinal analysis with time and group as within- and between-subjects factors, respectively. For post hoc multiple comparisons, the Bonferroni method was used. A P value < 0.05 was considered to be statistically significant.
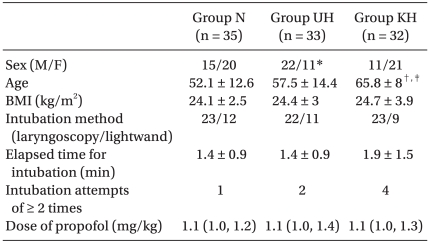
Patient characteristics, intubation-related data and drug dosage are summarized in Table 1. More male patients were recruited to Group UH (P < 0.01, compared to Group KH). Patients in Group KH were older than those in Groups N and UH (P < 0.001 and P < 0.05, respectively). The proportion of lightwand to laryngoscopy use, and the weight-standardized dose of propofol, as well as elapsed time and number of attempts for intubation, were similar between the three groups. No patients presented with an ST change or dysrhythmia during induction or tracheal intubation.
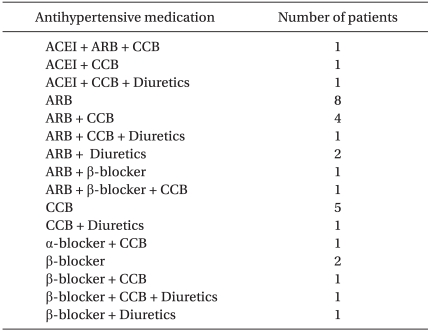
The antihypertensive medications used in Group KH are listed in Table 2. Angiotensin receptor blockers were the most frequently used agents, followed by calcium channel blockers. More than half of the patients were taking at least two different agents simultaneously (12 patients took two different drugs, and five patients took three different drugs).
Numerical data of hemodynamic parameters over nine time points are presented in Table 3. The baseline HR was similar in all three groups. Baseline arterial pressures, however, were higher in Groups UH and KH than in Group N (P < 0.001, except for DAP in Group KH, P = 0.002). Moreover, the baseline DAP and MAP in Group UH were greater than in Group KH (P = 0.001, and P = 0.009, respectively).
After induction of anesthesia, HR and arterial pressures significantly decreased below the baseline values in all three groups (P < 0.001). With tracheal intubation, HR significantly increased over the baseline values in all three groups (P < 0.001). Within 5 min after tracheal intubation, HR gradually decreased to the baseline levels in Groups UH and KH. In Group N, however, HR remained higher than baseline value until 5 min after tracheal intubation (P = 0.008). Immediately after tracheal intubation, arterial pressures increased over the baseline values in all three groups. However, only DAP and MAP in Groups N and UH were significantly greater than baseline for 1 min after intubation (P < 0.001 and P = 0.003 for DAP, and P = 0.001 and P = 0.031 for MAP, respectively). Thereafter, arterial pressures decreased below the baseline values in all three groups (P < 0.01). Theses serial changes in HR and MAP are depicted in Fig. 1 as SAP and DAP changed in parallel to MAP over time. Changes in HR were not influenced by group discrimination. However, MAP changes over time in Group UH were significantly different from those in Group N (P < 0.001).
Compared among groups, HR and arterial pressures in Groups UH and KH remained higher than those in Group N immediately after induction: in Group UH, HR and SAP for 1 min (P = 0.023 and P < 0.001, respectively), and DAP and MAP for 2 min (P = 0.004); in Group KH, SAP and MAP for 1 min (P < 0.001, and P = 0.008, respectively). Thereafter, HR and arterial pressures all became comparable among groups immediately before intubation. After tracheal intubation, HR increased over the baseline values, but was comparable among groups. Arterial pressures in Group UH were greater than in Group N (P = 0.015, P = 0.023, and P = 0.008 for SAP, DAP, and MAP, respectively). Moreover, MAP in Group UH was higher than in Group KH (P = 0.035). These increases in arterial pressures were observed for 1 min after tracheal intubation and then disappeared, which was comparable among groups by 5 min after tracheal intubation, except that MAP in Group UH was significantly higher than in Group N at 4 min after intubation.
Rescue therapy was given in 13 patients (two in Group N, eight in Group UH, and three in Group KH) without significant differences among groups. Most of the rescue therapy was to treat hypertension after intubation (one in Group N, seven in Group UH, and two in Group KH): only one patient in Group N presented with hypotension requiring ephedrine administration.
In this study, we demonstrated that a low-dose regimen of remifentanil, consisting of a 0.5 µg/kg bolus followed by continuous infusion of 0.1 µg/kg/min, given before induction of anesthesia with bolus propofol, resulted in similar hemodynamic responses to induction and tracheal intubation in patients with diverse cardiovascular conditions. Although the untreated hypertensive patients were shown to have statistically different hemodynamic responses from those in normotensive patients with relatively larger amplitude of pressure swing, this brief but exaggerated response clinically means nothing but requirement for additional rescue therapy for those patients. Furthermore, the low-dose regimen of remifentanil was also shown to be effective at stabilizing hemodynamics before intubation, and at limiting pressor responses to tracheal intubation without excessive cardiovascular depression.
Remifentanil is considered to present the ideal pharmacological profile to treat potentially adverse hemodynamic responses to the noxious but brief stimulus of tracheal intubation. Earlier investigations with a remifentanil bolus with or without continuous infusion demonstrated that remifentanil effectively mitigated or even abolished those responses [15-17]. However, much higher doses of remifentanil were used than in our study and these higher doses of remifentanil were frequently (up to 50%) associated with bradycardia and/or hypotension, especially if no pretreatment with glycopyrrolate was performed [15-17]. Hall et al. [11] demonstrated that a 0.5 µg/kg bolus of remifentanil, followed by an infusion of 0.25 µg/kg/min, was as effective as a higher (double) dose at attenuating pressor responses to laryngoscopy and tracheal intubation. Furthermore, a low-dose remifentanil regimen, identical to that used in our study was shown to be as effective as an equipotent dose of alfentanil to attenuate hemodynamic responses to tracheal intubation in hypertensive patients [12]. In these previous investigations done in westerners, the low-dose regimen of remifentanil produced a similar pattern of hemodynamic responses to tracheal intubation: HR and arterial pressures decreased after induction of anesthesia, then increased with tracheal intubation without exceeding baseline values [11,12,15-18]. In the investigations done in Koreans, however, greater sensitivity to remifentanil, given either by bolus or infusion, was demonstrated with no further increases in hemodynamic responses even after intubation [13,14]. Therefore, we chose this lowest effective dosage of remifentanil to evaluate the efficacy of a lower dose of remifentanil to treat hemodynamic responses to tracheal intubation. Hemodynamic responses in our study showed a similar pattern to that reported in the previous investigations, except for elevations in HR and arterial pressures over baseline values immediately after intubation. These relatively larger increments in hemodynamic parameters can be attributed to vagolytic pretreatment for prevention of bradycardia and/or hypotension with remifentanil [11,16], along with the fact that we did not give volatile anesthetics during mask ventilation before tracheal intubation. Although hemodynamic variables were elevated over the baseline values in all groups, patients in Group KH showed no statistically significant increases, which was probably attributed to a certain stabilizing effect of ongoing antihypertensive therapy. Elevated HR and arterial pressures quickly decreased to below the baseline values; even though HR in Group N remained higher than baseline until 5 min after intubation without clinical relevance (HR values were < 90/min). It is encouraging that in our study, a low-dose, single regimen of remifentanil produced similar hemodynamic responses to induction and tracheal intubation in patients with diverse cardiovascular conditions. Although MAP changes over time in the untreated hypertensive patients were shown to statistically different from those in normotensive patients with relatively larger amplitude of pressure swing, this exaggerated response to induction and intubation was so brief (< 1-2 min) that clinically it only necessitates an adequate rescue regimen for hemodynamic stabilization in those patients. Adverse events, such as hypotension and/or bradycardia, were observed in fewer cases compared to previous investigations (about 1% vs. 10%, respectively) [11,15]. In our study, rescue medications were mostly used to treat hypertension after tracheal intubation, more frequently in Group UH. Inadequate protection against hyperdynamic responses to intubation might be a concern. These augmented hemodynamic responses, however, were short-lived and well-controlled with predetermined rescue medications. Furthermore, twice the number of hypertension and/or tachycardia incidents during induction and intubation were reported in the multicenter study by Hogue et al. [18] compared with our study (10% vs. 21% for hypertension, respectively), in which twice the remifentanil dosage used in our study was employed as a "small dose". To summarize, a low-dose regimen of remifentanil in this study can stabilize hemodynamics before intubation and limit hemodynamic responses to tracheal intubation without excessive cardiovascular depression.
Our study has certain limitations. First, disparities between patients in terms of their characteristics and baseline hemodynamic profiles may nullify the assumption of randomization. However, this disparity is likely to be a reflection of epidemiologic properties of hypertension; for example, the prevalence of hypertension increases with advanced age, and women have been shown to have better awareness, treatment and control of hypertension [19]. To achieve hemodynamic stability during induction of anesthesia, target-controlled infusion would be a better choice than weight-adjusted bolus administration [20]. When designing the study, however, our primary goal was to emulate as closely as possible the ordinary routine practice of anesthesia. A variety of antihypertensive medications were used in Group KH, making it difficult to evaluate the effect of individual class of those agents, due to low statistical power. Although pressor responses to intubation are reportedly not affected by antihypertensive agents [21], this must be evaluated in the future in hypertensive patients who are administered novel antihypertensive agents. As hemodynamic and neuroendocrine responses to tracheal intubation have been shown to be limited for 5 min [22], we designed our study accordingly. However, it is important to evaluate whether the hemodynamic responses measured for "several minutes" after intubation could affect perioperative outcomes [23]. As mentioned above, greater sensitivity to remifentanil in Koreans was demonstrated in the investigations in Koreans [13,14] and could be extrapolated from the similarity of ED50 of remifentanil for awake fiberoptic intubation reported in patients of similar East Asian descent [24]. However, further randomized study in large numbers of patients is required for clarification.
In conclusion, a low-dose regimen of remifentanil in our study limited pressor responses to intubation effectively, and produced similar hemodynamic responses among various groups of patients without excessive cardiovascular depression. However, the relatively larger alterations in hemodynamic responses observed in our study indicate the necessity of developing supplementary regimens to control those exaggerated responses, especially in the untreated hypertensive patients. Thus, we suggest that low-dose regimen of remifentanil in our study could be commonly used to modify hemodynamic responses to tracheal intubation in patients with various hemodynamic characteristics if adequate rescue therapy is readily prepared.
References
1. Kovac AL. Controlling the hemodynamic response to laryngoscopy and endotracheal intubation. J Clin Anesth 1996; 8: 63-79. PMID: 8695083.


2. Prys-Roberts C, Greene LT, Meloche R, Foëx P. Studies of anesthesia in relation to hypertension. II. Hemodynamic consequences of induction and endotracheal intubation. Br J Anaesth 1971; 43: 531-547. PMID: 5089931.



3. Randell T, Seppälä T, Lindgren L. Isoflurane in nitrous oxide and oxygen increases plasma concentrations of noradrenaline but attenuates the pressor response to intubation. Acta Anaesthesiol Scand 1991; 35: 600-605. PMID: 1785237.


4. Dahlgren N, Messeter K. Treatment of stress response to laryngoscopy and intubation with fentanyl. Anaesthesia 1981; 36: 1022-1026. PMID: 7032347.


5. Crawford DC, Fell D, Achola KJ, Smith G. Effects of alfentanil on the pressor and catecholamine responses to tracheal intubation. Br J Anaesth 1987; 59: 707-712. PMID: 3111508.



6. Stoelting RK. Attenuation of blood pressure response to laryngoscopy and tracheal intubation with sodium nitroprusside. Anesth Analg 1979; 58: 116-119. PMID: 571234.


7. Ebert JP, Pearson JD, Gelman S, Harris C, Bradley EL. Circulatory responses to laryngoscopy: the comparative effects of placebo, fentanyl and esmolol. Can J Anaesth 1989; 36: 301-306. PMID: 2566391.


8. Glass PS, Hardman D, Kamiyama Y, Quill TJ, Marton G, Donn KH, et al. Preliminary pharmacokinetics and pharmacodynamics of an ultra-short-acting opioid: remifentanil (GI87084B). Anesth Analg 1993; 77: 1031-1040. PMID: 8105723.


9. Egan TD, Lemmens HJ, Fiset P, Hermann DJ, Muir KT, Stanski DR, et al. The pharmacokinetics of the new short-acting opioid remifentanil (GI87084B) in healthy adult male volunteers. Anesthesiology 1993; 79: 881-892. PMID: 7902032.


10. Westmoreland CL, Hoke JF, Sebel PS, Hug CC Jr, Muir KT. Pharmacokinetics of remifentanil (GI87084B) and its major metabolite (GI90291) in patients undergoing elective inpatient surgery. Anesthesiology 1993; 79: 893-903. PMID: 7902033.


11. Hall AP, Thompson JP, Leslie NA, Fox AJ, Kumar N, Rowbotham DJ. Comparison of different doses of remifentanil on the cardiovascular response to laryngoscopy and tracheal intubation. Br J Anaesth 2000; 84: 100-102. PMID: 10740557.



12. Maguire AM, Kumar N, Parker JL, Rowbotham DJ, Thompson JP. Comparison of effects of remifentanil and alfentanil on cardiovascular response to tracheal intubation in hypertensive patients. Br J Anaesth 2001; 86: 90-93. PMID: 11575417.



13. Gwak MS, Choi SJ, Yoon JS, Lee JY, Yang M, Kim GS, et al. Hemodynamic responses to rapid sequence endotracheal intubation using propofol and rocuronium at three different doses of remifentanil infusion. Korean J Anesthesiol 2006; 50: 385-389.

14. Cha JW, Kwak SH, Kim SJ, Choi JI, Kim CM, Jeong ST, et al. Optimal dose of remifentanil to blunt hemodynamic response to laryngoscopy and endotracheal intubation during induction of anesthesia with propofol. Korean J Anesthesiol 2006; 51: 292-296.

15. McAtamney D, O'Hare R, Hughes D, Carabine U, Mirakhur R. Evaluation of remifentanil for control of hemodynamic response to tracheal intubation. Anaesthesia 1998; 53: 1223-1227. PMID: 10193231.


16. Thompson JP, Hall AP, Russell J, Cagney B, Rowbotham DJ. Effect of remifentanil on the hemodynamic response to orotracheal intubation. Br J Anaesth 1998; 80: 467-469. PMID: 9640152.


17. O'Hare R, McAtamney D, Mirakhur RK, Hughes D, Carabine U. Bolus dose remifentanil for control of hemodynamic response to tracheal intubation during rapid sequence induction of anesthesia. Br J Anaesth 1999; 82: 283-285. PMID: 10365011.



18. Hogue CW Jr, Bowdle TA, O'Leary C, Duncalf D, Miguel R, Pitts M, et al. A multicenter evaluation of total intravenous anesthesia with remifentanil and propofol for elective inpatient surgery. Anesth Analg 1996; 83: 279-285. PMID: 8694306.


19. Cutler JA, Sorlie PD, Wolz M, Thom T, Fields LE, Roccella EJ. Trends in hypertension prevalence, awareness, treatment, and control rates in United States adults between 1988-1994 and 1999-2004. Hypertension 2008; 52: 818-827. PMID: 18852389.


20. De Castro V, Godet G, Mencia G, Raux M, Coriat P. Target-controlled infusion for remifentanil in vascular patients improves hemodynamics and decreases remifentanil requirement. Anesth Analg 2003; 96: 33-38. PMID: 12505919.


21. Sear JW, Jewkes C, Tellez JC, Foëx P. Does the choice of antihypertensive therapy influence hemodynamic responses to induction, laryngoscopy and intubation? Br J Anaesth 1994; 73: 303-308. PMID: 7946853.



22. Kayhan Z, Aldemir D, Mutlu H, Oğüş E. Which is responsible for the hemodynamic response due to laryngoscopy and endotracheal intubation? Catecholamines, vasopressin or angiotensin? Eur J Anaesthesiol 2005; 22: 780-785. PMID: 16211744.


23. Thomson IR. The hemodynamic response to intubation: a perspective. Can J Anaesth 1989; 36: 367-369. PMID: 2758536.


24. Xu YC, Xue FS, Luo MP, Yang QY, Liao X, Liu Y, et al. Median effective dose of remifentanil for awake laryngoscopy and intubation. Chin Med J (Engl) 2009; 122: 1507-1512. PMID: 19719938.

Fig. 1
Line-scatter plots (A and B) depict the alterations in heart rate (HR) and mean arterial pressure (MAP), respectively, over nine time points during induction of anesthesia and tracheal intubation, showing intra-group comparisons in Group N (●), Group UH (○), and Group KH (▾). Values are mean ± SD. Group N: normotensive, Group UH: untreated hypertensive, Group KH: known hypertension. *P < 0.01, compared to baseline, †P < 0.001, compared to baseline, ‡P < 0.05, compared to baseline.

Table 3
Hemodynamic Data During Induction of Anesthesia and Tracheal Intubation Showing Comparisons Among Groups

Values are mean ± SD. HR: heart rate, SAP: systolic arterial pressure, DAP: diastolic arterial pressure, MAP: mean arterial pressure. Group N: normotensive, Group UH: untreated hypertensive, Group KH: known hypertension. *P < 0.05, compared to Group N, †P < 0.001, compared to Group N, ‡P < 0.01, compared to Group KH, §P < 0.01, compared to Group N, ∥P < 0.05, compared to Group KH.
- TOOLS