The prophylactic use of recombinant factor VIIa in a patient with DeBakey type III aortic dissection -A case report-
Article information
Abstract
Little is known about the prophylactic use of recombinant factor VIIa (rFVIIa) in patients undergoing surgery for a bleeding aorta employing cardiopulmonary bypass. We report the successful use of rFVIIa in a patient undergoing hypothermic circulatory arrest and prolonged cardiopulmonary bypass for repair of a DeBakey type III aortic dissection.
Recombinant factor VIIa (rFVIIa) was developed to treat bleeding episodes in patients with hemophilia A or B who have inhibitors of factor VIII or IX and in patients with acquired hemophilia. At present, rFVIIa is also used for hemostatic resuscitation of severe trauma patients, and those with refractory bleeding during cardiac surgery [1-3] and postoperative hemorrhage. The use of rFVIIa as a prophylactic agent has been limited, because of concerns about the possibility of thrombosis and because of the high cost of rFVIIa. Thus, little is known about the prophylactic use of rFVIIa in patients undergoing surgery for an aortic hemorrhage [4]. We describe here the prophylactic use of rFVIIa in a patient undergoing hypothermic circulatory arrest and prolonged cardiopulmonary bypass for repair of a DeBakey type III aortic dissection.
Case Report
A 62-year-old man (175 cm, 68 kg), confirmed as having aortic dissection type III, presented for aortic surgery. Computed tomography showed that the dissection would extend from his subclavian artery to the level of his renal artery.
Before the induction of general anesthesia, an intra-arterial catheter was inserted into his radial artery under local anesthetic. General anesthesia was induced with etomidate 12 mg iv and rocuronium 50 mg iv and maintained with a continuous infusion of propofol (effect site concentration 1.0-2.0 ug/ml) and remifentanil (effect site concentration 9-16 ng/ml) using a target controlled infusion pump (Orchestra® Base Primea, Fresenius Vial, Brezins, France). Hypnotic depth was assessed using a bispectral index monitor (BIS, A-2000, Aspect Medical Systems, Natick, MA, USA). A continuous infusion of rocuronium 30 mg/h was started. After tracheal intubation, with a double lumen tube for thoracotomy, his lungs were mechanically ventilated with 100% oxygen. Lumbar drainage was inserted at L3-4 level to drain cerebrospinal fluid (CSF), thus controlling intracranial pressure and maintaining CSF pressure at 8 to 10 mmHg. After iv injection of heparin 2000 IU, a central venous catheter (Arrow-HowesTM Large-Bore, Arrow® International Inc, Reading, PA, USA) and a superior vena cava cannula (DLP™ Femoral Arterial Cannula, 17 Fr, Medtronic Inc, Minneapolis, MN, USA) were inserted through the right subclavian vein and right internal jugular vein, respectively. A second central venous catheter (Arrow-Howes™, 7.5 Fr, 3 Lumen, Arrow® International Inc, Reading, PA, USA) was inserted through the left subclavian vein. We monitored five-lead electrocardiography, pulse oximetry, capnography, urine output, electroencephalography (EEG) and cerebral oximetry (SOMANETICS® INVOS OXIMETER, Troy, MI, USA). A cell salvage device (AUTOLOG™, Medtronic Inc, Minneapolis, MN, USA) was used, and salvaged blood was reinfused before the end of surgery.
During cardiopulmonary bypass (CPB), activated clotting time (ACT) was maintained at >480 seconds with an initial heparin dose of 300 IU/kg and 3 additional doses of total 16,000 IU each. At a systemic temperature of 17.5℃, achieving a flat EEG and a bispectral index of 0, total circulatory arrest was allowed for 18 minutes. Total CPB time was 248 minutes, and cross-clamp time was 75 minutes. During CPB, the patient received 1,500 ml of plasma as priming solution, followed by 1,400 ml packed red blood cells (P-RBC), 3,000 ml of plasma and 1,000 ml of half-saline, administered by a CPB machine. After uneventful weaning from CPB, the patient was administered 4.5 mg/kg of protamine to reverse the effects of heparin and return the ACT to its preoperative level, 122 seconds.
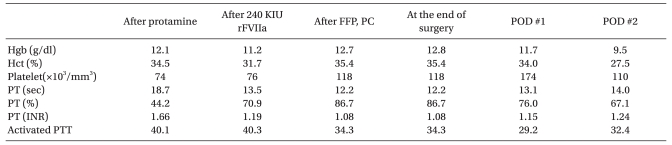
At this point, his prothrombin time (PT) was 18.8 seconds, his international normalized ratio (INR) was 1.66 and his activated partial thrombin time (aPTT) was 40.1 seconds. Complete blood count (CBC) showed that his hemoglobin concentration was 12.1 g/dl and his platelet count was 74,000/mm3. Rotational thromboelastometry (ROTEM Analyser, Tem International GmbH, Munich, Germany) showed a clotting time (CT) of 359 seconds, a clot formation time (CFT) of 290 seconds, an α angle of 47°, a maximum clot firmness (MCF) of 43 mm, and a maximum lysis (ML) of 100% (Fig. 1). Ten minutes after prophylactic administration of 240 kIU (4.8 mg) rFVIIa, his PT was 13.5 seconds, his INR was 1.19 and his aPTT was 40.3 seconds. ROTEM showed a CT of 216 seconds, a CFT of 257 seconds, an α angle of 49°, an MCF of 43 mm, and an ML of 18% (Fig. 1). Before obtaining the latter set of coagulation results, we had administered 2 units of P-RBC, 5 units of FFP, and 10 units of platelet concentrates to treat the anticipated coagulopathy. No additional rFVIIa or blood products were required. At the end of surgery, CBC showed a hemoglobin concentration of 12.7 g/dL and a platelet count of 118,000/mm3. Coagulation profile revealed a PT of 12.2 seconds, an INR of 1.08 and an aPTT of 34.3 seconds (Table 1). The total surgical time was 602 minutes; fluids administered included 1,500 ml normal saline, 1,500 ml synthetic colloid, 2 units of P-RBC (plus 7 units during CPB), 5 units of FFP, 10 units of PC, 1,300 ml saved blood, and 800 ml pump blood. Urine output was 1,950 ml, and expected blood loss was 3,780 ml.

ROTEM (INTEM). (A) After protamine administration. CT 359, CFT 290, α 47°, MCF 43 mm, ML 100%. (B) After rFVIIa administration. CT 204, CFT 267, α 48°, MCF 43 mm, ML 23%. CT: clotting time. CFT: clot formation time. α: α-angle. MCF: maximum clot firmness. ML: maximum lysis.
In the ICU, no blood products were required to maintain hemoglobin or coagulation level. During the first few hours, the chest tubes drained 50-130 ml/h, later decreasing to 0-30 ml/h. On postoperative day 1, the patient was weaned from the ventilator. On postoperative day 2, however, the patient's mental state was drowsy and he was reintubated due to poor expectoration. At that time, CBC revealed a hemoglobin concentration of 11.7 g/dl and a platelet count of 174,000/mm3. His PT was 13.1 seconds, his INR was 1.15 and his aPTT was 29.2 seconds. On postoperative day 3, the patient's mental state became alert, allowing extubation. The patient was moved to the general ward on postoperative day 6 and discharged, with no complications, on postoperative day 17.
Discussion
Most repairs of aortic dissection involve large amounts of bleeding, despite improvements in surgical technique. Bleeding results from various interrelated factors, including the extent of surgical dissection, the use of anticoagulant for CPB, ischemia and reperfusion, fibrinolysis and dilution of platelets and coagulation factors. In this patient, the aortic dissection was widely extended, and both CPB and deep hypothermic circulatory arrest (DHCA) were required. We therefore decided to use rFVIIa to prevent large amounts of bleeding. In most centers, however, rFVIIa is used primarily when standard coagulation interventions have failed to control blood loss [2,5-7], even in the absence of definitive guidelines for the use of rFVIIa in cardiac surgery.
Prophylactic rFVIIa has also been used in individuals with normal coagulation profiles who underwent retropubic prostatectomy with an expectation of massive perioperative bleeding [8]. That study showed that an injection of rFVIIa could reduce perioperative blood loss and eliminate the need for transfusion in patients undergoing major surgery. Prophylactic use of rFVIIa during orthotopic liver transplantation was found to significantly reduce the number of patients requiring RBC transfusion, with no increase in thromboembolic events compared with placebo [9]. In addition, rFVIIa was used to manage hemostasis in a Jehovah's Witness [4].
Recombinant FVIIa was originally developed to manage bleeding in patients with hemophilia. In normal individuals, <1% of plasma rFVII is in the form rFVIIa. When rFVIIa is administered, it complexes with tissue factors at the injured vascular site; the resulting complex, in turn, initiates the coagulation cascade. rFVIIa also directly activates factor X on the surfaces of activated platelets independent of factor VIII and IX. The main hemostatic effect of rFVIIa is to increase the rate of thrombin generation. Therefore, rFVIIa can improve coagulation profiles and clot formation time. Indeed, we found that the coagulation profiles in our patient improved before administration of plasma products and platelets (Table 1).
Many patients with massive bleeding suffer from fibrinolysis due to consumption of fibrinogen. Although aprotinin can reduce fibrinolysis, this agent is rarely used in cardiac surgery due to its association with high rates of renal failure, myocardial infarction or heart failure and stroke or encephalopathy [10]. rFVIIa was found to simultaneously accelerate clot formation and inhibit fibrinolysis by activating thrombin activatable fibrinolysis inhibitor (TAFI). Therefore, rFVIIa seems to eliminate the need for an additional antifibrinolytic agent such as aprotinin, tranexamic acid and aminocaproic acid. In our patient, ROTEM showed dramatically improved maximum lysis, from 100% to 23%, in the INTEM test.
Prior to administration of rFVIIa, fibrinogen concentration should be >50 mg/dl, platelet count should be >50,000/mm3 and pH should be ≥7.1. At the time of rFVIIa administration to our patient, pH was 7.28, while platelet count, INR and aPTT were within their recommendation ranges (Table 1). Independent predictors of failure to respond to rFVIIa were found to include pretreatment abnormal coagulation tests (INR > 2.0, aPTT > 60 seconds, fibrinogen <100 mg/dl or platelet count < 80,000) [3], although others have reported that pH, platelet level, and fibrinogen level were not associated with response [2].
The most serious complications of rFVIIa are thromboembolic. These complications are very rare in hemophilia patients and those with congenital or acquired factor VIII or IX inhibitors [11]. Most patients who undergo aortic dissection are atherosclerotic. They may have vulnerable plaques especially in coronary and cerebral arteries, which can trigger the extrinsic coagulation pathway during surgery. Therefore, if rFVIIa acts locally, ischemic events can activate these arteries. Monocytes are activated during and after CPB, and these monocytes are the main source of TF expression [12,13]. Adding rFVIIa may result in both local and systemic TF activation and finally promote thrombotic complications.
Despite concerns about thrombosis, rFVIIa has some benefits in cardiac surgery. These include improvements in coagulation profiles, reduced volumes of transfusion products and transfusion related complications, and rescue hemostasis in case of intractable bleeding during cardiac surgery. Although rFVIIa is being used primarily when standard interventions have failed to control blood loss [3], prophylactic use of rFVIIa may have efficacy and safety. Another issue related to rFVIIa is the cost-effectiveness in its off-label use. The official price for 120 kIU of rFVIIa is £1410. Although rFVIIa is cost-effective in hemophilia patients [14,15], the cost effectiveness of its off-label use in cardiac surgery has not been determined, especially as a prophylactic agent.
In conclusion, we found that prophylactic use of rFVIIa in a patient undergoing aortic dissection resulted in a favorable outcome and safety of the operation. This single case report, however, could not determine whether use of rFVIIa in a major operation is more beneficent or harmful. That determination requires large-scale controlled clinical trials to evaluate the efficacy and safety of this treatment.