|
 |
|
|
Abstract
Background
This study was performed to find the optimal volume of local anesthetics needed for a successful ultrasound-guided stellate ganglion block (SGB) to treat head and neck pathology.
Methods
Fifteen female and fourteen male sensory-neural hearing loss patients received 4 times SGBs with 0.2% ropivacaine in volumes of 6, 4, 3 and 2 ml at 1 to 3 day intervals. Using the transverse short-axis view of the neck that showed Chassaignac's tubercle at the C6 level, a 25-gauge, and 4 cm needle was inserted via the lateral paracarotid approach with out-of-plane targeting between the prevertebral fascia and the ventral surface of longus colli muscle (subfascial injection). A successful block was confirmed with the onset of ptosis (Horner's syndrome).
Results
There were no significant statistical differences between the presence of Horner's syndrome and the volume of local anesthetics given. However, Horner's syndrome was present in all trials for the 4 ml and 6 ml groups. Six (20.7%) and three out (10.4%) of twenty-nine trials in the 2 ml and 3 ml groups, respectively, failed to elicit Horner's syndrome. The duration of action was significantly different in the 2 ml group compared to that of the 6 ml group, but there was no significant difference between the other groups, including the 4 ml vs. 6 ml groups. The side effects were not different between the groups.
Several studies have focused on the optimal amount of local anesthetics needed for a successful stellate ganglion block (SGB). However, the previous studies were conducted by performing SGBs under the blind technique [1-5]. Furthermore, the reported minimum or optimal requirements of local anesthetics were controversial in the previous studies; 5 ml of 1% mepivacaine [5], 8 ml of 0.2% ropivacaine [2], 8 ml of 0.5% lidocaine [3], or 6 ml of 1% lidocaine for head and neck disease and above 12 ml of 1% lidocaine for upper extremity disease [4]. Cho et al. [2] compared 8 ml of 1% lidocaine, 0.2% bupivacaine and 0.2% ropivacaine when used for SGB, and they reported 0.2% ropivacaine was in the middle of these agents when its pharmacological properties were compared to the faster onset and short duration of lidocaine and the slowed onset and long duration of bupivacaine.
We have used 6 ml of 0.2% ropivacaine for SGB in patients with a head and neck pathology in daily practice for several years based upon the results of previous studies [1-4], and we have recently adopted ultrasound imaging in daily practice for performing SGB.
The ultrasound guidance technique for SGB was first published a case series by Kapral et al. [6] in 1995, but this technique has only recently gained popularity. Kapral et al. [6] found that ultrasound-guided SGB, as compared with the blind technique, used a lower volume of local anesthetics (5 ml rather than 8 ml) and there was a more rapid onset of Horner's syndrome. Narouze et al. [7] also used 5 ml of 0.25% bupivacaine for ultrasound-guided SGB. Application of ultrasound for regional nerve blockade has proven to be useful for the swift and accurate placement of local anesthetics on a targeted area [8], and it might reduce the required volume of local anesthetics as compared with that of the blind technique.
Therefore, this study was performed to find the optimal volume of 0.2% ropivacaine for a successful ultrasound-guided SGB for head and neck pathologies in daily practice.
After approval by the Institutional Review Board, 29 patients with sensory-neural hearing loss and who were referred to the pain clinic for SGB were selected. Fifteen women and fourteen men with a median age of 55.1 years (range: 38-73) were included in this study (Table 1). All the patients were ASA Physical Status 1 or 2 and they had no history of anticoagulant or coagulopathy.
All the procedures were performed by the same anesthesiologist for a technical consistency. A double-blind, randomized study for the volume was conducted in which 0.2% ropivacaine was given to the patients. Each SGB was allocated into one of four different volume (6 ml, 4 ml, 3 ml or 2 ml) groups. Each patient received 4 SGBs, which were done by repeated trials using a randomized volume of the four different groups of 0.2% ropivacaine, with a washout interval of 1 to 3 days.
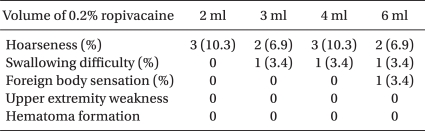
The patients were positioned the same as for the classical blind technique, with the neck extended and this was aided by a 15 cm pillow placed under the shoulders, and the mouth slightly open to relieve tension of the neck. With the help of an ultrasound imaging device with a 13-16 MHz linear array probe (HFL38x, Sonosite M-Turbo®; Sonosite, Bothell, WA), which provided a transverse short-axis view of the neck and this showed the prominent anterior tubercle of the C6 transverse process (Chassaignac's tubercle) at the C6 level. The thyroid gland, the carotid artery, the compressible internal jugular vein, the prevertebral fascia (PVF), Chassaignac's tubercle and the oval shaped structure of the longus colli muscle were revealed on this short-axis view (Fig. 1). The color Doppler mode was used before needling to avoid penetrating blood vessels such as a vertebral artery, the inferior thyroid vessels and etc. A 25-gauge, 4 cm needle (Kovax®) was advanced at the lateral paracarotid side with the out-of-plane approach, and the needle was inserted parallel and 0.2 cm apart from the probe on the skin. The needle tip was inserted at the middle of the lateral side of the carotid artery and Chassaignac's tubercle, and then it penetrated through the completely compressed internal jugular vein to the target point under the PVF and above the ventral surface of the longus colli muscle (a subfascial injection). All the procedure was continued real-time with visualization of the needle tip as an echogenic dot on the screen. Finally, the correct position of the needle was confirmed by the expansion of local anesthetics beneath the PVF and above the longus colli in the transverse view (Fig. 2). Injections were permitted after a negative aspiration test, and following injection of 0.2 ml of local anesthetics to rule out intravascular placement of the needle. Every injection was divided by less than 1 ml and the needle was repositioned several times to keep the exact position of the needle under direct visualization to prevent displacement of the needle and overshooting the injectate. After removal of the needle, manual compression of the injection site for 5 min was done to prevent hematoma formation. Thirty minutes later, the patient's neck was re-examined with ultrasound to check for hematoma formation before his/her discharge.
The time of injection was recorded at the time of needle removal, and the onset of Horner's syndrome, which was assessed by checking for signs of ptosis, was checked every minute thereafter. The time to relief of ptosis was recorded by the patient who was instructed to check every 30 minutes. The duration action of the local anesthetic was counted from the time of needle removal to the time of the relief of ptosis.
Side effects were recorded upon recognition by the doctor during the patient's recovery state in the clinic. The common side effects were checked, including hoarseness, dysphagia and a foreign body sensation in the throat, upper extremity weakness, and hematoma formation. The patients were instructed to inform the doctor of any adverse symptoms they experienced upon their next visit.
The data was analyzed using SPSS version 15.0 (SPSS Inc., Chicago, IL, USA) and the results were expressed as means ± SDs. To compare the differences between the groups, Fisher's exact tests were used for the rates of occurrence of ptosis and side effects after SGB, and Kruskal-Wallis test was used for the analysis of the onset time and the duration of action. P value less than 0.05 was considered statistically significant.
Positive cranial sympathetic blockade (Horner's syndrome) following ultrasound-guided SGB was present in all the trials in the 4 ml and 6 ml groups with using 0.2% ropivacaine (Table 2). Six (20.7%) and 3 (10.4%) out of the twenty-nine trials in 2 ml and 3 ml groups respectively, failed to elicit Horner's syndrome. However, there were no statistically significant differences between the presence of Horner's syndrome and the volume of local anesthetics given (P > 0.05, Fisher's exact test). Also, the onset time was not statistically different between the four groups (Table 2, P > 0.05, Kruskal-Wallis test).
The action duration was 134.3 ± 44.1, 158.5 ± 64.5, 165.5 ± 60.3 and 197.6 ± 87.6 min in the 2 ml, 3 ml, 4 ml and 6 ml groups, respectively (Table 2). The duration of action was significantly different between the 2 ml and 6 ml groups (P < 0.001, Kruskal-Wallis test), but the duration of action was not significantly different between the 2 ml vs. 3 ml, 2 ml vs. 4 ml, 3 ml vs. 4 ml, 3 ml vs. 6 ml and 4 ml vs. 6 ml groups (P > 0.05).
The side effects were revealed as follows. Hoarseness was the most common side effect (Table 3). The incidences of the hoarseness were 10.3%, 6.9%, 10.3% and 6.9% in the 2 ml, 3 ml, 4 ml and 6 ml groups, respectively, without significant differences between the groups. Dysphagia was noted in one case each in the 3 ml, 4 ml and 6 ml groups (each 3.4%), and a foreign body sensation was revealed in only one case in the 6 ml group (3.4%). All the groups were similar for being free of upper extremity weakness and hematoma formation. Although diverse side effects were seen, there were no statistically significant differences between the groups (Table 3, P > 0.05, Fisher's exact test).
To find the optimal volume of 0.2% ropivacaine required for a successful ultrasound-guided SGB for head and neck pathology, we performed a double blind randomized study on 29 patients with sensory-neural hearing loss. All the patients received four times ultrasound-guided SGBs separately using a volume of four different volumes of 0.2% ropivacaine. All the patients in the 4 ml and 6 ml groups displayed Horner's syndrome, while some of the patients in the 2 ml and 3 ml groups failed to elicit Horner's syndrome. Furthermore, there were no differences in the onset time, the duration of action and the incidence of the side effects in the 4 ml and 6 ml groups. Therefore, the authors of this study recommend that 4 ml is the optimal volume of 0.2% ropivacaine for performing an ultrasound-guided SGB for the patients with head and neck pathologies.
Stellate ganglion blocks are performed for the purpose of blocking the sympathetic innervations of the cranial and cervical nerves and sometimes the nerves of the upper extremities. Sympathetic outflow from the white rami communicants of the upper thoracic nerves enter the corresponding sympathetic ganglia and then they ascend into the neck, where they are fused into ganglions: the superior, middle, inferior and the cervicothoracic ganglions. Among them, the cervicothoracic ganglion (a fusion of the inferior cervical and first thoracic ganglions), which is also called stellate ganglion, was not the target for blockade in this study. Only the blockade of the middle or inferior cervical ganglion was needed to promote blood flow to the cranial area for our patients with acute sensory-neural hearing loss, and to elicit Horner's syndrome. Horner's syndrome (ptosis, miosis and enophthalmos), as well as ipsilateral flushing of the conjunctiva and skin, blocking of the nose and anhydrosis, are the result when the cervical sympathetic fibers are successfully blocked [9]. The development of Horner's syndrome is usually taken to mean that an accurate SGB for the head and neck area has been done, but not for the upper extremity [9,10]. It has been used as an indicator for successful cervical sympathetic block [2,3,5,6,9-11]. Furthermore, ptosis is the most common sign (100%) after SGB [11]. We used the development of Horner's syndrome (and especially ptosis) as an indicator for successful cervical sympathetic block in this study. Although the location or even the presence of the stellate ganglion varies among patients, numerous trials [1-7] have shown that SGB performed at the level of the C6 transverse process is sufficient for the blockade of the sympathetic innervations of the cranial area, and we also performed US-guided SGB at the level of the C6 tubercle in this study.
Many imaging devices have been studied for enhancing the accuracy of SGBs to prevent the serious side effects such as epidural [12], subarachnoid [13] and intra-arterial injections [14,15]. Among these, computerized tomography [16] and MRI [17] are considered to be time-consuming and expensive, and most of all they are impractical for most practitioners [18]. Fluoroscopy has gained popularity for its convenience and the relatively familiar real-time imaging, but fluoroscopy still cannot prevent improper injections into other important anatomical structures, such as the esophagus, which may be accidentally punctured and this can lead to infection of the neck [7,19].
The ultrasound guidance technique was first published in a case series by Kapral et al. [6] in 1995, but it has only recently gained popularity. They found that ultrasound-guided SGB, compared with the blind technique, used a lower volume of local anesthetics (5 ml rather than 8 ml) and it showed a more rapid onset of Horner's syndrome. Narouze et al. [7] also used 5 ml of 0.25% bupivacaine for ultrasound-guided SGB. Application of ultrasound for regional nerve blockade has proven to be useful for the accurate placement of local anesthetics on a targeted area [8], and it might reduce the volume of local anesthetics compared with that of the blind approach.
In this study, the lateral paracarotid out-of-plane technique was used when performing an ultrasound-guided SGB. The out-of-plane injection technique used in this study was different from the lateral paracarotid in-plane injection technique reported by Gofeld et al. [20]. The insertion point of the needle was at the middle between the lateral side of the carotid and the medial border of Chassaignac's tubercle, and the target area was into just beneath the PVF and above the longus colli muscle (a subfascial injection). Therefore, the needle passed through the compressed internal jugular vein (Fig. 2). With this technique, repeated injections precisely at the level of C6 were possible by confirmation of the subfascial expansion during injection (Fig. 2). Two concerns are possible with using this technique. The first is the needle path penetrating the internal jugular vein. We were concerned about this before our trials, but it might not be problematic if the procedure is carefully performed because the vein was completely compressed during procedure and no hematoma formation occurred following the procedure. The second is the concern about the out-of-plane approach. There is controversy about the in-plane vs. out-of plane needle approaches for ultrasound-guided nerve blocks [21,22]. The pros and cons for both methods have been debated and there is no data showing that one is better than the other. The method is generally chosen according to the operator's preference. Although out-of-plane is criticized because of the poor visualization of the needle tip and its related complications, the operator's skill is the most important variable that influences the needle's visibility [21]. Actually in this study, we carefully performed out-of-plane approaches with special attention including that the needle was inserted at 0.2 cm apartand perpendicularly down at nearly parallel to the probe (the ultrasound beam) to get better visualization, and the probe was intermittently angled during the procedure for real-time confirmation of the needle tip with the echogenic dot disappearing distal to it [22]. Further details about the technique are not needed for this volume study.
There is agreement that larger volumes (more than 10 ml) of local anesthetics for SGB are needed for blockade of the upper extremity pathologies [1,23]. However, the optimal volume for the head and neck is still controversial. Large volumes exceeding 10 ml of local anesthetics were not needed in this study for the purpose of cranial sympathetic blockade in patients with sudden sensory-neural hearing loss. Kapral et al. [6] and Narouze et al. [7] used 5 ml of local anesthetics for ultrasound-guided SGB. Lee et al. [5] recommended 5 ml, and others [1-4] have recommended 6-8 ml as the optimal volume for SGB using the classical blind technique.
In a preliminary study, we experienced that only 1 or 2 ml of 0.2% ropivacaine was enough for ultrasound-guided SGB in some patients. However, the optimal volume in daily practice means that there is no failure according to the small volume of local anesthetics injected into an exact targeted area. Also, in this study, ultrasound-guided SGBs were effectively performed even with 2 ml in some patients, but not in all. The failure rate of the 2 ml groups was 20.7%. It means that 2 ml of local anesthetics is not optimal in daily practice. Although the success rates were not significantly different between the four groups, both the 2 ml (79.3%) and 3 ml (89.6%) groups did not reach 100%, which is unlike the 4 ml and 6 ml groups. Furthermore, the duration of action was significantly different between the 2 ml and 6 ml groups (P < 0.001, Kruskal-Wallis test), but there was no significant difference between the 4 ml and 6 ml groups (P > 0.05). Therefore, 4 ml of 0.2% ropivacaine, instead of 6 ml, was more optimal for successful SGB, and 2 or 3 ml, which was expected to be optimal before the study, was not sufficient for routine SGB. Our data showed that 4 ml was an optimal volume for ultrasound-guided SGB, and this volume was lower than those previously reported volumes of the blind technique (5-8 ml) [1-5] and it was also a little lower than previously reported volumes for ultrasound-guided SGB (5 ml) [6,7]. By the way, 4 ml as an optimal volume for an ultrasound-guided SGB was larger than we expected before the study. The cause of the required larger volume might be the lack of the pressure effect as compared with that of blind injection, which is performed in the compressed space around the longus colli muscle. Lee et al. [24] reported digital compression during injection for SGB with the blind technique affected the spread of local anesthetics; 6 ml with digital compression showed a similar increase of skin temperature compared with that for 10 ml without digital compression.
There were several side effects following SGB such as hoarseness, a foreign body sensation and dysphagia in all the groups, and the occurrence rate was considerably lower than those of the previous studies that used larger volumes of local anesthetics. However, our study failed to show a comparable difference of the rate of occurrence of side effects between the groups. Although there was a three-fold difference of the volume between the minimum (2 ml) and maximum (6 ml) volume administered, the rate of occurrence of side effects was low in all four groups, without relevance to the volume. The ultrasound-guided technique may also have contributed to the low rate of side effects due to its ability to help accurately inject into the target space and thereby prevent accidental injection.
Several studies with volumes larger than 5 ml have shown the spread of the injectate into distant areas with reaching several nervous structures such as the carotid baroreceptor and the vagus nerve [25,26]. Thus, hemodynamic regulation may be hindered [27]. Severe hypertension may be elicited by the unopposed cardiac sympathetic stimulation by blockade of the vagus nerve [28,29]. However, hypertension may be induced even by a small volume of local anesthetics. Kimura et al. [29] reported seven cases of severe hypertension after SGB using 5 ml of 1% mepivacaine. As we have not experienced severe hypertension in our facility using 0.2% ropivacaine for all our SGBs, the type or the permeability of the local anesthetics used may be more of importance rather than the amount [30]. These differences of local anesthetics might require further studies.
In conclusion, this SGB volume study shows that large volumes of local anesthetics are not required when performing ultrasound-guided SGB. Although the 2 ml group in this study showed as effective as those of the 4 ml and 6 ml groups without any statistically significant difference, the 2 ml and 3 ml groups showed a meaningful failure rate. Therefore, we recommend that the optimal volume of 0.2% ropivacaine for ultrasound-guided SGB to treat head and neck pathology in daily practice is 4 ml. Two ml or 3 ml are not optimal for a routine SGB unless further large scale studies support using lower volumes.
References
1. Song SO, Jo YW. Effects of the volume of local anesthetic used in stellate ganglion block on the elevation of skin temperature of ipsilateral upper extremity. Korean J Anesthesiol 1999; 37: 233-239.

2. Cho YW, Song SO, Jang JH. Effect of stellate ganglion block using 0.2% ropivacaine. J Korean Pain Soc 2000; 13: 182-186.
3. Chang JH, Song SO. Minimal concentration of lidocaine for a diagnostic stellate ganglion block. Korean J Anesthesiol 2001; 41: 165-170.

4. Song SO, Suh YH. Changes of plasma lidocaine concentrations after stellate ganglion block according to volume-changes of 1% lidocaine. J Korean Pain Soc 2001; 14: 26-31.
5. Lee HK, Chung SY, Yang SK, Lee HJ, Suh YS, Kim C. Minimal volume of local anesthetic for successful stellate ganglion block. J Korean Pain Soc 1995; 8: 60-64.
6. Kapral S, Krafft P, Gosch M, Fleischmann D, Weinstabl C. Ultrasound imaging for stellate ganglion block: direct visualization of puncture site and local anesthetic spread. A pilot study. Reg Anesth 1995; 20: 323-328. PMID: 7577781.

7. Narouze S, Vydyanathan A, Patel N. Ultrasound-guided stellate ganglion block successfully prevented esophageal puncture. Pain Physician 2007; 10: 747-752. PMID: 17987096.


8. Koscielniak-Nielsen ZJ. Ultrasound-guided peripheral nerve blocks: what are the benefits? Acta Anaesthesiol Scand 2008; 52: 727-737. PMID: 18477070.


9. Breivik H, Cousins MJ, Lofstrom JB. Edited by Cousins MJ, Bridenbaugh POSympathetic neural blockade of upper and lower extremity. Neural blockade in clinical anesthesia and management of pain. 1998, 3rd ed. : Philadelphia, New York, Lippincott-Raven Publishers. pp 411-447.
10. Malmqvist EL, Bengtsson M, Sörensen J. Efficacy of stellate ganglion block: a clinical study with bupivacaine. Reg Anesth 1992; 17: 340-347. PMID: 1363053.

11. Son MK, Chung RK, Kim YJ, Kim DY, Lee HS, Han JI. The effects of local anesthetic distribution on symptoms using ultrasound image after stellate ganglion block. Korean J Anesthesiol 2009; 57: 579-583.


12. Makiuchi T, Kondo T, Yamakawa K, Shinoura N, Yatsushiro K, Ichi S, et al. Stellate ganglion blocks as the suspected route of infection in a case of cervical epidural abscess. No Shinkei Geka 1993; 21: 805-808. PMID: 8377897.

13. Wulf H, Maier C. Complications and side effects of stellate ganglion blockade. Results of a questionnaire survey. Anaesthesist 1992; 41: 146-151. PMID: 1570888.

14. Ellis JS Jr, Ramamurthy S. Seizure following stellate ganglion block after negative aspiration and test dose. Anesthesiology 1986; 64: 533-534. PMID: 3963469.

15. Park KH, Song SO. Convulsion after stellate ganglion block with 0.2% ropivacaine: a case report. Korean J Anesthesiol 2003; 45: 536-539.

16. Erickson SJ, Hogan QH. CT-guided injection of the stellate ganglion: description of technique and efficacy of sympathetic blockade. Radiology 1993; 188: 707-709. PMID: 8351337.


17. Hogan QH, Erickson SJ, Haddox JD, Abram SE. The spread of solutions during stellate ganglion block. Reg Anesth 1992; 17: 78-83. PMID: 1581263.


18. Abdi S, Zhou Y, Patel N, Saini B, Nelson J. A new and easy technique to block the stellate ganglion. Pain Physician 2004; 7: 327-331. PMID: 16858470.


19. Moore DC. An unusual complication after stellate ganglion block. Br J Anaesth 1964; 36: 601PMID: 14207660.



20. Gofeld M, Bhatia A, Abbas S, Ganapathy S, Johnson M. Development and validation of a new technique for ultrasound-guided stellate ganglion block. Reg Anesth Pain Med 2009; 34: 475-479. PMID: 19920422.


21. Schafhalter-Zoppoth I, McCulloch CE, Gray AT. Ultrasound visibility of needles used for regional nerve block: an in vitro study. Reg Anesth Pain Med 2004; 29: 480-488. PMID: 15372394.


22. Chapman GA, Johnson D, Bodenham AR. Visualisation of needle position using ultrasonography. Anaesthesia 2006; 61: 148-158. PMID: 16430568.


23. Moore DC. Therapeutic stellate ganglion block: 5 versus 10 ml of a local anesthetic. Reg Anesth Pain Med 2008; 33: 191-192. PMID: 18299102.


24. Lee JW, Son JS, Kim SK, Kim YY, Choe H, Han YJ. Evaluation of the spreading effect of injection volume due to use of the modified injection technique in stellate ganglion block. Korean J Anesthesiol 2008; 54: 43-46.

25. Hardy PA, Wells JC. Extent of sympathetic blockade after stellate ganglion block with bupivacaine. Pain 1989; 36: 193-196. PMID: 2919100.


26. Guntamukkala M, Hardy PA. Spread of injectate after stellate ganglion block in man: an anatomical study. Br J Anaesth 1991; 66: 643-644. PMID: 2064877.


27. Taneyama C, Goto H. Fractal cardiovascular dynamics and baroreflex sensitivity after stellate ganglion block. Anesth Analg 2009; 109: 1335-1340. PMID: 19762767.


28. Yokota S, Komatsu T, Kimura T, Shimada Y. A case of severe hypertension caused by stellate ganglion block in a patient with facial palsy. Masui 1996; 45: 1123-1126. PMID: 8905949.

29. Kimura T, Nishiwaki K, Yokota S, Komatsu T, Shimada Y. Severe hypertension after stellate ganglion block. Br J Anaesth 2005; 94: 840-842. PMID: 15849210.



30. Wulf H, Maier C, Schele HA, Wabbel W. Plasma concentration of bupivacaine after stellate ganglion blockade. Anesth Analg 1991; 72: 546-548. PMID: 2006745.


Fig. 1
Sonoanatomy for an ultrasound-guided SGB: the transverse short-axis view at the C6 level. CA: carotid artery, IJV: internal jugular vein (in a partially compressed state by the probe), PVF: prevertebral fascia, LCo: longus colli muscle, SCM: sternocleidomastoid muscle, Thy: thyroid gland, T: Chassaignac's tubercle, med: medial, lat : lateral.
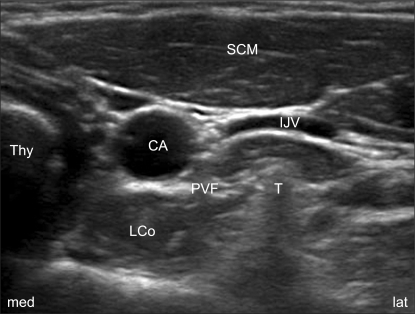
Fig. 2
Ultrasound image of the C6 transverse short-axis view following left SGB at the C6 level. Arrows show the subfascial spread of local anesthetics above the longus colli muscle. The fine long arrow indicates the out-of-plane needle path. CA: carotid artery, LCo: longus colli muscle, SCM: sternocleidomastoid muscle, Thy: thyroid gland, T: Chassaignac's tubercle, med: medial, lat: lateral.
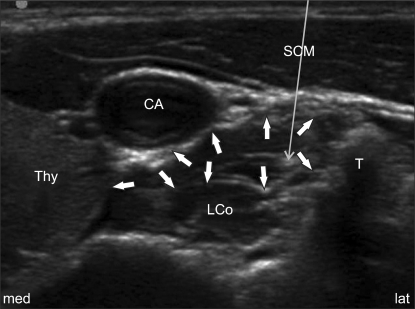
Table 2
Comparison of the Four Different Volumes of 0.2% Ropivacaine used for an Ultrasound-guided SGB (N = 29)

Values are means ± SDs. *The 2 ml and 3 ml groups appeared to have a lower incidence rate of Horner's syndrome than those of the 4 ml and 6 ml groups, but there was no significant difference (P > 0.05). †The duration of action was significantly different between the 2 ml and 6 ml groups (P < 0.001, Kruskal-Wallis test), but it was not significantly different between the other groups (P > 0.05). SGB: stellate ganglion block.
- TOOLS