The relationship between the predictors of obstructive sleep apnea and difficult intubation
Article information
Abstract
Background
The study was performed in order to determine the risk factors for difficult tracheal intubation in obstructive sleep apnea patients.
Methods
For 115 male patients with obstructive sleep apnea syndrome and who were undergoing palatal muscle resection (PMR), we investigated the correlation between their age, height, weight, body mass index (BMI), their Epworth Sleepiness Scale (ESS), their apnea-hypopnea index (AHI), their neck circumference and the difficulty of tracheal intubation.
Results
The factors significantly related to difficult tracheal intubation in obstructive sleep apnea patients were a high AHI and a large neck circumference. There was no significant correlation between weight, BMI, arterial hypertension, ESS and difficult tracheal intubation in obstructive sleep apnea patients.
Conclusions
In this study, a high AHI and a large neck circumference can predict difficult tracheal intubation in obstructive sleep apnea patients.
Introduction
Obstructive sleep apnea syndrome (OSAS) is the next most common disorder after asthma [1], it causes sleep disorders and hypoxia because of the trembling of the pharyngeal wall and palate when inspiring due to the repetitive, partial or complete obstruction of the pharynx [2]. Without proper sleep, the patient may experience excessive daytime sleepiness (EDS), headache, change in behavior, reduced intelligence and even personality change [3]. Furthermore, it may be the cause of severe complications such as hypertension, pulmonary hypertension, cardiac arrhythmia and congestive heart failure [4-6].
OSAS is commonly accompanied by factors such as the thickening of the tonsil and adenoid, obesity and mandibular hypoplasia, and the upper airway obstruction may worsen in such cases [4]. When inducing general anesthesia during palatal muscle resection (PRM) [6] to treat such OSAS patients, and the maintenance of mask ventilation may be difficult due to the airway obstruction from the reduced pharyngeal muscle tension [7,8], and intubation may be difficult due to the abnormalities of the face or the upper airway [9]. Such a difficult intubation can be the main factor leading to increased morbidity and an increased death rate [10,11].
We report here on the effects of the predictors of OSAS (which is the morbidity due to hypertension, habitual snoring, apnea observed during sleep, choking during sleep, the abnormal structure of the upper airway, a thick neck circumference and moderate obesity [12,13]) according to the difficulty of performing intubation for general anesthesia.
Materials and Methods
One hundred fifteen ASA class I & II male patients were chosen for this study and they were diagnosed with sleep apnea in the Departments of Internal Medicine and ENT and they underwent PMR under general anesthesia because they failed to respond to medicinal treatment. The authors obtained the permission from the hospital's ethics committee and we obtained informed written consent from the patients after the objectives of the study were explained to them. Before the surgery, the patient's age, height, weight and level of obesity (as the body mass index [BMI]) were recorded. The neck circumference was recorded at the level of the thyroid cartilage. A polysomnography was performed on all the patients for one night. Apnea was defined as when respiration completely stopped for 10 sec or more while there was still respiratory movement. Hypopnea was defined as at least a 50% decrease of the respiration amplitude, and this was accompanied by a clear decrease of the tidal volume for at least 10 sec, EEG arousal and a decrease in oxygen saturation by 4% or more [14]. The apneahypopnea index (AHI) is the mean sum of the occurrences of apnea and hypopnea for every hour of sleep where an AHI < 5 is for patients who only snore and who do not complain of excessive daytime sleepiness (EDS) during the day: 5 ≤ AHI < 15 for the patients with mild sleep apnea, 15 ≤ AHI < 30 for the patients with moderate sleep apnea and an AHI ≥ 30 for the patients with severe sleep apnea [14]. Another method uses larger ranges as 5 ≤ AHI < 25 for the patients with mild sleep apnea, 25 ≤ AHI < 50 is for the patients with moderate sleep apnea and an AHI ≥ 50 is for patients with severe sleep apnea [15,16]. Before surgery the patients were asked to complete the AHI and Epworth Sleepiness Scale (ESS) (Table 1). The ESS is a subjective scale where the patient grades his excessive daytime sleepiness (EDS) from 0 to 3 in 8 specific situations, such as while sitting and reading a book or watching TV. If the total score for the 8 situations is 10 or higher, then the patient is considered to have EDS [17].
On the day the nocturnal polysomnography (NPSG) was taken, if the systolic blood pressure, which was taken 3 times, was 140 mmHg or above or if the diastolic blood pressure was 90 mmHg or above, then the patient was considered to have hypertension. After the patient had been lying down and was stabilized, the blood pressure was taken 3 times with 10 minute intervals.
No premedication was given that had sedatives. Thirty minutes prior to entering the OR, 0.2 mg IM glycopyrrolate was administered. Standard EKG electrodes, a noninvasive automatic blood pressure monitor and a pulse oximeter were placed. After waiting 5 minutes for the patient to stabilize, the blood pressure and pulse rate were taken before anesthetic induction. After the patient was seated comfortably, the patient was made to sit up and sick out his tongue as far as he could in order to observe the internal structure of the pharynx under lighting. Mallampati et al. [18] classified the pharyngeal structure into 3 types, but Samsoon and Young [19] created a modified Mallampati test (MMT) that divides the structure into 4 types. Class I is when the soft palate, the fauces, the uvula and the anterior and posterior tonsillar pillars are visible. Class II is where all the Class I structures are visible except for the tonsillar pillars. Class III is where only the base of the uvula is visible. Class IV is where the uvula cannot be seen and only the soft palate is visible.
Anesthesia was performed by a skilled anesthesiologist. For denitrogenization, 100% oxygen 5 L/min for 2-3 min was given by a mask and this was aspired by voluntary respiration. Propofol (10 mg/ml) and remifentanil (50 µg/ml) were administered by a TCI pump (OrchestraTM, Fresenius Vial, France). The Minto model was chosen for remifentanil and the Schnider model was chosen for propofol. Propofol was set at a target effect site concentration of 4.0 µg/ml. Remifentanil was set at a target effect site concentration of 3.0 ng/ml. Propofol was administered after administering remifentanil and the target effect site concentration reached 3.0 ng/ml. After loss of consciousness was observed in the patient, 0.6 mg/kg rocuronium (a muscle relaxant) was administered IV. After about 2 minutes when the state of muscle relaxation was observed, the head was placed in the sniffing position to facilitate intubation. Then a size-3 curved laryngoscope was used to maximize exposure of the glottis, and without pressing the thyroid cartilage, the airway was evaluated and graded following Cormack and Lehane's grading system [20]. Cormack and Lehane proposed grading the laryngoscopic view from grade I to grade IV. Grade I is when the epiglottis and vocal cords are completely exposed. Grade II is when only the rear of the vocal cords can be seen. Grade III is when only the epiglottis is exposed. Grade IV is when only the soft palate can be seen. After intubation, mechanical ventilation was performed with oxygen at 2 L/min, carbon dioxide at 2 L/min, the tidal volume was 9 ml/kg and the tidal rate was 12 bpm.
Group E, for whom the intubation was easy, was limited to the patients with a Cormack and Lehane grade of I and II (the glottis was immediately visible). Group D, for whom the intubation was difficult, was for the patients given a Cormack and Lehane grade of III or IV (poor visibility of the glottis) [21]. All the values are shown as means ± SDs and as percentages. Independent samples t-tests, crosstabulation and chi square tests were used for comparing the two groups. P values < 0.05 were considered significant. Ultimately, a comprehensive evaluation was done by binary logistic regression analysis and a multivariate test to see the effects of each independent variable on the dependent variables.
Results
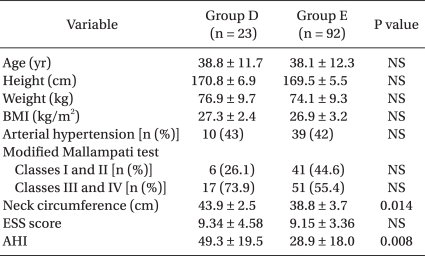
The test results showed that of the 115 OSAS patients who underwent PMR, Group E consisted of 92 patients (80%) and Group D consisted of 23 patients (20%). There were no significant differences in the age, height, weight, BMI, hypertension or the rate of the prevalence among the OSAS patients (Table 2).
Among the OSAS patients, the patients with MMT Classes III and IV made up 73.9% of Group D, which was greater than the 55.4% in Group E. The neck circumference in Group D was 43.9 ± 2.5 cm, which was a larger value than 38.8 ± 3.7 cm in Group E (P = 0.014) (Table 2).
The AHI, which shows the severity of OSAS, was 49.3 ± 19.5 for Group D, and this was greater than 28.9 ± 18.0 for Group E (P = 0.008). However, there was no significant difference found in the ESS score, which is a subjective test for sleep apnea (Table 2).
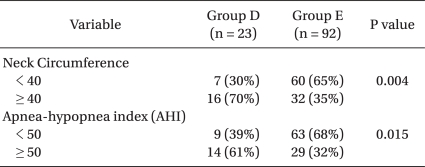
Seventy percent of the Group D patients had neck circumferences ≥ 40 cm, whereas only 35% of the Group E patients had neck circumferences ≥ 40 cm (P = 0.014). Also, 61% of the Group D patients had an AHI ≥ 50, but only 32% of the Group E patients had an AHI ≥ 50 (P = 0.038) (Table 3).
The results of the logistic regression analysis showed that for the sleep apnea patients, the AHI and neck circumference were the variables affecting difficult intubation (P < 0.05). As the AHI increased, the probability of belonging to Group D went up 1.124 times (the 95.0% confidence interval of the odds ratio was 1.036 and 1.221) (Table 4). As the neck circumference increased, the probability of belonging to Group D went up 1.764 times (the 95.0% confidence interval of the odds ratio was 1.290 and 2.411) (Table 4). Thus, the variables affecting the probability of belonging to Group D were a high AHI and large neck circumference, and the neck circumference had the highest effect.
Discussion
Charles Kite was the first to describe intubation with difficult airway maintenance in 1788 [22]. Since then, there have been ongoing developments in the methods of intubation. Yet difficult intubation in general anesthesia has remained a main concern for anesthesiologists. Difficult intubation is especially frequent in OSAS patients [23,24] and it is one of the main causes of increased morbidity and mortality rates [10,11].
The first reason that difficult intubation occurs in OSAS patients is that many OSAS patients have retrognathia, short and thick necks, and frequent abnormal facial and upper airway structures such as a large tongue, which all increase the danger of difficult intubation [9]. Of the OSAS patients in the present study and in whom intubation was difficult according to the Cormack and Lehane grading, there were 21 grade III (18.3%) patients and 2 patients grade IV (1.7%) patients. In the presented study, one of the two Group D patients with a Cormack and Lehane Grade of IV had successful intubation after the cricoid cartilage area was pressed and 1-3 attempts were made with a probe. A light wand was used in one patient with a Cormack and Lehane Grade of IV, and intubation was successful after 4 attempts. Lewis et al. [21] stated that intubation is difficult in patients with a Cormack and Lehane grade of III and IV, which is defined based on the exposure of the larynx. Further, one report stated that grade III patients may face great difficulty to be intubated, and in the grade IV patients, intubation is complicated without the help of special aiding methods [25]. Therefore, before general anesthesia, analyzing and predicting the risk factors that affect difficult intubation is critical for OSAS patients. For the patients who are suspected of having sleep apnea, a thorough evaluation of the upper airway at a pre-surgery visit or before anesthesia induction must be performed, and evaluation of the predictors of OSAS must be performed first. According to the other reports on this subject, hypertension, habitual snoring, apnea during sleep, choking during sleep, an anthropometrical upper airway structure, a thick neck circumference and moderate obesity are known to be significant predictors for OSAS [26]. One study reported there is also a significant relationship between the BMI and OSAS [27].
The second reason that difficult intubation occurs in OSAS patients is due to their short necks and obesity [28]. Difficult intubation occurs when the BMI is high, the neck circumference is large and the Mallampati score is ≥ 3 [29]. The level of obesity can be assessed from the BMI. The BMI of an average man is around 22 and the BMI for the average woman is around 20. A man with a BMI of ≥ 27 is considered obese [30]. The authors found the BMIs of 115 patients were on average 27.0 ± 2.7, which showed that most of the OSAS patients belonged to the obese category [3,29]. Yet among the OSAS patients, there was no significant difference in the BMI of the patients with difficult intubation compared to the BMI of the patients without difficult intubation. Although obesity may be related to difficult intubations [31], since most of the OSAS patients were obese, it is assumed that the difference in BMI among the OSAS patients did not act as a significant risk factor affecting the difficulty of intubation.
In a report based on patients in the West, the danger of OSAS was reported to be high when the neck circumference for men was ≥ 43 cm and that for women was ≥ 40 cm [32]. However, for Asians, in contrast to the rule above, the occurrence rate of OSAS significantly increases if the neck circumference is ≥ 40 cm irrespective of gender [26]. The neck circumference of Group D in the present study was 43.9 ± 2.5 cm and that for Group E was 38.8 ± 3.7 cm. For patients with difficult intubation, their neck circumferences were significantly thicker (P = 0.014). Moreover, 70% of the patients with difficult intubations had neck circumferences ≥ 40 cm. Only 35% of the patients with easy intubation had a neck circumference ≥ 40 cm. Thus, the factor that most influenced the difficulty of intubations in OSAS patients was the thickness of the neck. Forty cm or above can especially be considered a significant risk factor.
The severity of OSAS, the AHI (which can be determined via polysomnography) and the subjective ESS score are also known to be main predictors of OSAS [23]. In the present study, difficult intubation in OSAS patients and a high AHI were correlated. Sixty one percent of the moderate sleep apnea patients who had an AHI of ≥ 50 had difficult intubations and only 32% had easy intubations (Table 3). Therefore, for OSAS patients scheduled for PMR and who have an AHI ≥ 50 (which is for severe sleep apnea), thorough preparation prior to anesthesia must be done for difficult intubation and other related risks.
However, the ESS score, which is a patient-subjective sleep apnea test, did not show a significant relation with difficult intubation in OSAS patients. This may be because of the subjectivity involved and the difficulty for patients to objectively measure themselves may have created errors. This lack of significance may have resulted from an anatomically abnormal upper airway having less direct relevance as compared to that of the AHI.
Predicting difficult intubation and airway management is necessary. For average patients, the BMI and weight are known to be predictive factors for difficult intubation, but their significance as predictors in the present study was low. However, even in the OSAS patients, the predictors of difficult intubation were a high Mallampati score and a high AHI, and the neck circumference also worked as a risk factor for difficult intubation. If the Mallampati score is ≥ 3, the AHI is ≥ 50 and the neck circumference is ≥ 40 cm, then it can be predicted that intubation will be difficult, so thorough preparations must be made for such cases.
First, one must be careful in choosing the drugs for anesthesia induction. Most OSAS patients are sensitive to the sedatives used for anesthesia induction. In anesthesia induction, the worst case scenario for an anesthesiologist can occur, such as when ventilation does not work and intubation is impossible, so it is recommended to use drugs with a short onset time. Also, depending on the situation, intubation using awake fiberoptic bronchoscopy and retrograde intubation can be useful in OSAS patients [5].
Second, steroids can be preventively used to avoid airway obstruction that can occur after surgery. Preparation for emergency bronchotomy along with the use of a monitoring device such as a pulse-oximeter and an oxygen supply of the appropriate concentration would be advisable.
A high AHI (which is most closely-related to the severity of OSAS), polysomnography and a thick neck can be the predictors of difficult intubation in OSAS patients. Polysomnography and the neck thickness can be obtained from the basic medical records of OSAS patients. Especially for patients in whom PMR is performed, polysomnography has become mandatory. So observing the upper airway evaluations, the AHI (which reflects the severity of OSAS) and the neck thickness can help predict difficult intubation.