|
 |
|
|
Abstract
Background
Propofol is used for supraglottic airway device insertion, often with the i-gel. However, the propofol requirement for i-gel insertion has not been explored in paralyzed patients. This study was performed to explore hemodynamic changes and sedation level with different propofol doses in healthy paralyzed patients when the i-gel was inserted.
Methods
A total of 141 patients undergoing a urologic operation were randomly allocated to three groups depending on the propofol dose (1.5, 2, and 2.5 mg/kg; Groups P1.5, P2, and P2.5, respectively). After patients had been administered each propofol dose and rocuronium, the i-gel was inserted and changes in hemodynamic parameters and bispectral index were evaluated.
Results
Group P2 showed a lower incidence of complications (17%) such as hemodynamic instability and inadequate sedation than Group P1.5 (55.3%, P < 0.001) or Group P2.5 (40.4%, P = 0.012). The incidence and dose of additional propofol increased in Group P1.5 (51%, median [range]; 20 [0ŌĆō50]) compared with those in the other groups (0%, 0 [0ŌĆō0] in Group P2 and 8.5%, 0 [0ŌĆō50] in Group P2.5, all P < 0.001), and the incidence and dose of additional ephedrine were significantly higher in Group P2.5 (31.9%; 0 [0ŌĆō20]) than in Group P1.5 (10.6%, P = 0.012; 0 [0ŌĆō5], P = 0.007, respectively).
Supraglottic airway devices (SADs) such as laryngeal mask airway (LMA) ClassicŌäó, LMA ProSealŌäó, LMA Supreme┬«, and i-gel┬« can be used for emergency situations with failed tracheal intubation, as well as for elective inpatient or outpatient surgery under general anesthesia [1,2,3]. Despite the risk of gastric insufflation and regurgitation, SADs have advantages over tracheal intubation, including a decreased incidence of postoperative sore throat and coughing, improved hemodynamic stability, and reduced anesthetic requirements [4].
Propofol, the most commonly used intravenous anesthetic, is preferred for SAD insertion and ambulatory surgery using SADs due to its depressant effect on airway reflexes and its antiemetic effect [5].
The reported dose of propofol alone, without the use of pretreatments such as fentanyl, midazolam, or neuromuscular blocking agents, for insertion of LMA ClassicŌäó varies from 2.0 to 3.42 mg/kg [6]. However, previous studies have been performed without the use of neuromuscular blocking agents, and LMA ClassicŌäó was used to determine the dose of propofol required for smooth SAD insertion. In addition, few studies have evaluated the depth of sedation using the bispectral index (BIS) [7].
Among the SADs, i-gel is relatively new and composed of a soft non-inflatable cuff, a tube section including a connector, a gastric channel to introduce the gastric catheter, and an integral bite block. i-gel showed better hemodynamic stability during insertion, higher insertion success rate at first attempt, and a lower complication rate compared to LMA ProSealŌäó [8]. Suppression of gag reflex, coughing, and movement using neuromuscular blocking agents may improve SAD insertion, and could decrease propofol requirements for SAD insertion. Although i-gel and propofol with neuromuscular blocking agents are commonly used in patients undergoing general anesthesia, few studies have explored the appropriate induction dose of propofol that provides an adequate depth of anesthesia and hemodynamic stability in paralyzed patients for i-gel insertion.
Therefore, we designed this study to evaluate the effects of different doses of propofol for i-gel insertion on hemodynamic parameters and the depth of sedation in healthy paralyzed patients.
This prospective, randomized, double-blinded study was approved by the Institutional Review Board of our hospital, and written informed consent was obtained from each patient. Inclusion criteria were age 20ŌĆō65 years, American Society of Anesthesiologists physical status (ASA PS) IŌĆōII, and patients undergoing elective urologic surgeries with an anticipated duration of less than two hours under general anesthesia. Exclusion criteria were uncontrolled hypertension, obesity (body mass index > 30 kg/m2), and patients with pharyngeal pathology or risk of gastroesophageal regurgitation (e.g., pregnancy, hiatal hernia). Patients with baseline (pre-induction) systolic blood pressure (SBP) > 180 mmHg or heart rate (HR) > 100 beats/min and patients who required more than one attempt at i-gel insertion were also excluded. Patient randomization was performed using online randomization software (http://www.randomization.com) and patients were randomly allocated (allocation ratio 1 : 1 : 1) to receive one of three different doses of propofol; group P1.5, group P2, and group P2.5 patients received 1.5, 2, and 2.5 mg/kg propofol for the induction of anesthesia using i-gel, respectively.
All patients fasted for at least eight hours and arrived at the operating room with no premedication. Hypertensive patients continued to take antihypertensive medicine until the morning of the surgery. Before induction of anesthesia, pulse oximetry, electrocardiography, BIS (BIS VISTAŌäó monitor and a four-electrode sensor; Aspect Medical Systems, Norwood, MA, USA), and noninvasive blood pressure monitoring were started. Baseline (pre-induction) SBP, diastolic blood pressure (DBP), and mean blood pressure (MBP) were determined from the average of three consecutive readings taken after arrival at the operating room and before anesthesia induction. All patients were treated with four times of vital capacity breathing using a face mask with 100% oxygen (8 L/min) for preoxygenation. Anesthesia was induced with one of three doses of propofol (1.5, 2.0, or 2.5 mg/kg) according to the patient allocation. After confirming the loss of consciousness and eyelash reflex, all patients received intravenous rocuronium (0.6 mg/kg). Ninety seconds after the injection of rocuronium, i-gel┬« (Intersurgical Ltd., Wokingham, Berkshire, UK) that was lubricated on the sides and posterior surface with water-soluble lubricant was inserted. The size of the i-gel was chosen based on the patient's body weight and the manufacturer's recommendation [9]. After i-gel insertion, it was fixated after confirming the square shape of end-tidal CO2 (ETCO2), adequate tidal volume, and bilateral chest expansion when mechanical ventilation was applied. The study period was defined as from propofol injection to five minutes after the insertion of i-gel. During the study period, if the BIS value was greater than 65 (persisting > 2 min), which is considered inadequate sedation, 20ŌĆō30 mg of supplementary propofol was injected to prevent the possibility of recall, which was recorded. Hemodynamic parameters (SBP, DBP, MBP, and HR) and BIS value were measured at pre-induction, immediately before and after insertion of i-gel, and then every one minute until five minutes after i-gel insertion. After i-gel insertion, anesthesia was maintained with 1.5% sevoflurane and 50% nitrous oxide in oxygen at a flow rate 4 L/min until five minutes after i-gel insertion.
During the study period, hypotension (fall in SBP pressure > 30% of pre-induction SBP or SBP < 90 mmHg) was treated with intravenous ephedrine in 5 mg increments every minute until hypotension was corrected, while hypertension (increase in SBP > 30% of pre-induction SBP or SBP > 200 mmHg) was treated with 0.5 mg of nicardipine. In addition, bradycardia (HR < 50 beats/min) and tachycardia (HR > 120 beats/min) were treated with 0.5 mg of intravenous atropine and 10 mg of esmolol, respectively. Induction of anesthesia and anesthetic management were performed by the same anesthesiologist who was blinded for the study.
Based on preliminary data with 30 patients in each group, it was hypothesized that the incidence of complications (inadequate sedation, hypotension, hypertension, bradycardia, and tachycardia) would be 15% in Group P2 and 45% in Group P1.5 and Group P2.5. With a power of 0.8 and ╬▒-value of 0.05 (2-sided), 42 patients for each group were required using SigmaPlot 12.5 (Systat Software Inc., San Jose, CA, USA). Considering the potential dropout rate of 10%, 47 patients were enrolled in each group.
Data were analyzed using SPSS software (ver. 18.0 for Windows; SPSS Inc., Chicago, IL, USA). The normality of the distribution of continuous variables was analyzed using the Shapiro-Wilk test. Differences in continuous variables of demographic data were analyzed using one-way analysis of variance. Complications, which represent the total incidence of propofol addition and the administration of cardiovascular drugs, and doses of additional propofol and administered cardiovascular drugs were analyzed using the Kruskal-Wallis test followed by the Mann-Whitney U test when P < 0.05. For the Mann-Whitney U test, P < 0.017 (i.e., 0.05/3 = 0.017) was considered statistically significant. Categorical variables were analyzed using the Žć2-test or Fisher's exact test, as appropriate. Changes in hemodynamic variables (SBP, DBP, MBP, and HR) and BIS over time were assessed using two-way repeated-measures analysis of variance with Bonferroni correction. A two-tailed P < 0.05 was considered statistically significant.
A total of 176 patients were enrolled and 35 patients were excluded; thus, 141 patients were randomly allocated into one of three groups and completed this study (Fig. 1). Demographic data were comparable among the groups (Table 1).
The total incidence of complications (inadequate sedation, hypotension, hypertension, bradycardia, and tachycardia) was significantly lower in Group P2 (17%) than in Group P1.5 (55.3%, P < 0.001) and Group P2.5 (40.4%, P = 0.012) (Table 2).
The dose and incidence of additional propofol were significantly higher in Group P1.5 (median [range]; 20 [0ŌĆō50], 51%) than in Group P2 and Group P2.5 (0 [0ŌĆō0], 0% in Group P2.0 and 0 [0ŌĆō50], 8.5% in Group P2.5, all P < 0.001). The dose and incidence of administered ephedrine were significantly increased in Group P2.5 (0 [0ŌĆō20], 31.9%) compared with those in Group P1.5 (0 [0ŌĆō5], P = 0.007; 10.6%, P = 0.012, respectively). Nicardipine, atropine, and esmolol were comparable among all groups (Table 2).
Changes in SBP, DBP, MBP, and HR over time during the study period were significantly different among groups (P < 0.001, = 0.011, = 0.001, and = 0.024, respectively), but did not show a statistically significant difference between Groups P2 and P2.5. SBP was significantly higher in Group P1.5 than in Group P2 (P = 0.013). DBP and MBP were significantly higher in Group P1.5 than in Group P2 (P = 0.013 and 0.004, respectively) and Group P2.5 (P = 0.016 and 0.011, respectively). HR was significantly increased immediately and one minute after the insertion of i-gel compared with that at baseline in only Group P1.5 (P < 0.001 and 0.001, respectively) (Fig. 2). BIS was significantly decreased in Group P2 and Group P2.5 compared with that in Group P1.5 (both P < 0.001) (Fig. 3).
In this study, the demand for propofol for additional sedation was higher in Group P1.5 than in Group P2 and Group P2.5. The demand for ephedrine for the treatment of hypotension was higher in Group P2.5 than in Group P1.5. The incidence of complications that required medication was lower in Group P2 than in the other two groups, whereas it was comparable between Group P1.5 and Group P2. The decrease in complications suggests that an induction dose of 2 mg/kg propofol rather than 1.5 or 2.5 mg/kg is more appropriate to provide adequate sedation and hemodynamic stability for i-gel insertion using rocuronium. A previous study showed similar results, where using 2 mg/kg propofol was better than using 1.5 mg/kg or 2.5 mg/kg. However, because this previous report used a mixture of enflurane and N2O before LMA classic insertion, the insertion conditions and propofol dose may have been affected [6].
SADs were originally used to maintain the airway by nonanesthetists or paramedical staff in an emergency situation or cases of difficult intubation without a neuromuscular blocking agent, which induces respiratory depression [10]. Compared with endotracheal intubation, SADs have several advantages including reduced postoperative sore throat after general anesthesia and reduced anesthetic requirements leading to potential benefits, including improved hemodynamic stability, less postoperative nausea, and decreased vomiting. Although SAD insertion and maintenance are possible without a neuromuscular blocking agent, use of a neuromuscular blocking agent can improve surgical conditions. Moreover, considering that pulmonary aspiration can be prevented by increasing the sealing pressure of the device and through gastric catheter insertion via the gastric channel, SADs in combination with a neuromuscular blocking agent may be more commonly used in general anesthesia [11]. However, few studies have explored the propofol dose for maintaining stable hemodynamics and adequate sedation based on BIS in paralyzed patients when SADs are inserted. Thus, this study was performed to assess the appropriate propofol dosage for hemodynamic stability and adequate sedation with a neuromuscular blocking agent, especially when the i-gel is used.
BIS, which reflects head bio-signals and is expressed as a number from 100 (fully awake) to 0 (cortical silence), was approved by the Food and Drug Administration in 1996, and most general anesthesia is monitored using this system to evaluate sedation level. When using SAD insertion, most studies have used opioids to depress airway reflexes and patient movement. However, the electroencephalographic (EEG) depression of BIS requires almost five-fold of analgesic dose of opioid [12]. In the modern anesthetic environment, BIS is often used to monitor sedation level; if propofol or opioid is overdosed, there is a decrease in BIS and an increase in side effects, such as hemodynamic instability. When a neuromuscular blocking agent is not administered, BIS may be higher than normal general anesthesia ranges, even in deeply anesthetized patients. In particular, high electromyography (EMG) may lead to an overdose of hypnotic agents or anesthetics. Although the neuromuscular blocking agents used in this study may affect BIS, we used the BIS device which can monitor the EMG, and propofol was administered depending on the sedation level after confirming whether the cause of the BIS increase was the high EMG or not [12]. Accordingly, the use of neuromuscular blocking agents may minimize side effects resulting from an overdose of hypnotic agents in the case of anesthetizing while performing BIS monitoring.
i-gel┬« used in this study is a relatively new device that provides more hemodynamic stability than LMA ClassicŌäó, LMA ProSealŌäó, and LMA supreme┬« [13,14]. Therefore, this study was performed assuming that the propofol requirements would decrease compared with those in previous reports [15,16,17]. In a previous report, the ED50 of propofol with i-gel insertion was 2.02 mg/kg, but that study was performed after premedication with midazolam and fentanyl, which has an influence on conditions for i-gel insertion, in contrast to our study in which a neuromuscular blocking agent was used [15]. Fundamentally, the induction dose of propofol for intubation is 1ŌĆō2.5 mg/kg depending on the age [16]. Therefore, we determined propofol dose ranged from 1.5 to 2.5 mg/kg. Since hypnotic agents (excluding propofol) and anesthetic adjuvants such as opioid were not used before i-gel insertion, we hypothesized that there would not be any severe complications, even with a maximum dose for intubation. In addition, the minimum dose was 1.5 mg/kg considering that the induction dose of propofol for patients older than 60 is 1ŌĆō1.75 mg/kg [17].
Propofol has been used as an induction agent for the insertion of diverse SADs because of the decreased incidence of gag reflex and laryngospasm [18]. However, a propofol dose of 3.42 mg/kg was required as a sole anesthetic for successful LMA ClassicŌäó insertion without any movement, which can induce severe hypotension [19]. Thus, SAD insertion was performed with opioid, dexmedetomidine, and a neuromuscular blocking agent [20,21,22]. Under these conditions, one would expect a decrease in movement and improved insertion. Hypotension was observed even at a decreased propofol dose, and inadequate sedation was observed because of the decreased propofol requirements as the opioid dose increased [23]. A previous study explored the propofol requirement of LMA ClassicŌäó, showing that the propofol dose without fentanyl was 3.42 mg/kg and that with fentanyl was 1.42 mg/kg. It is important to note that systolic arterial blood pressure after LMA ClassicŌäó insertion significantly decreased in both groups, regardless of fentanyl administration [19]. Likewise, hypotension as a result of propofol administration was observed in our study, where opioid was not used. On the other hand, subjects who presented with hypertension were only 2 of 141 subjects, which received 1.5 mg/kg propofol. This suggests that when propofol is used, the administration of adjuvants such as opioids, adrenergic antagonists, or hypotensive agents may be unnecessary in paralyzed patients. Recently, although methods that improve insertion conditions and facilitate LMA ClassicŌäó insertion have been explored using dexmedetomidine, these conditions induced bradycardia (HR less than 45 beats/min) in 20% of subjects [21]. In our study, only one patient required atropine due to bradycardia (HR 47 beats/min). If our criteria were applied to the criteria (HR less than 45 beats/min) of previous reports [21], the bradycardia would not have occurred. Thus, the use of neuromuscular blocking agents can decrease propofol requirements and maintain hemodynamic stability compared with agents that affect the hemodynamic balance, such as opioids and dexmedetomidine. Rocuronium is one of the most commonly used neuromuscular blocking agents, and does not cause clinically significant hemodynamic instability [24]. The rocuronium dose for endotracheal intubation is generally 0.6ŌĆō1.2 mg/kg, which is two- to four-fold higher than the ED95 [25]. Considering the short duration of surgery, we injected 0.6 mg/kg rocuronium, which is the minimum intubation dose.
Recently, some studies have explored whether the propofol requirement for i-gel insertion would be decreased using a neuromuscular blocking agent. These reports examined LMA ClassicŌäó insertion using mivacurium and concluded that mivacurium could provide better insertion conditions and higher success rates, and could decrease propofol requirements [22]. Another study where LMA ProSealŌäó was inserted using rocuronium showed that rocuronium improves the success rate on the first attempt, with lower incidences of laryngospasm, hoarseness, and pharyngeal pain, as well as higher sealing pressure [26]. Nevertheless, propofol requirements that could maintain stable hemodynamics while inserting the i-gel under rocuronium have not been determined. In addition, if insertion conditions could be improved by rocuronium and increases in propofol alone do not have a positive effect on insertion conditions [7], the optimal dose of propofol that can minimize hemodynamic changes while maintaining adequate sedation should be explored. In our study, we assessed the propofol dose that could maintain hemodynamic stability and adequate sedation using rocuronium which does not strongly affect hemodynamic stability [24] in comparison with previous reports that assessed success rates and insertion conditions without simultaneously measuring hemodynamic parameters and sedation level [8,9,13,14,18,19,23,26].
There are some limitations to our study. First, although invasive hemodynamic monitoring is required to detect subtle hemodynamic changes, we did not perform these procedures because they are excessive and unnecessary for ASA class I and II patients. It should also be noted that this study was performed by dividing into three groups according to different doses of propofol. Therefore, in the future, a dose-finding study to determine the optimal dose of propofol for stable hemodynamics and adequate sedation of SAD insertion is required when using rocuronium.
In conclusion, 2 mg/kg propofol may be used for i-gel insertion in healthy paralyzed patients compared to 1.5 mg/kg or 2.5 mg/kg to provide both hemodynamic stability and proper sedation.
References
1. Choi CG, Yang KH, Jung JK, Han JU, Lee CS, Cha YD, et al. Endotracheal intubation using i-gel® and lightwand in a patient with difficult airway: a case report. Korean J Anesthesiol 2015; 68: 501-504. PMID: 26495062.



2. Park SY, Rim JC, Kim H, Lee JH, Chung CJ. Comparison of i-gel® and LMA Supreme® during laparoscopic cholecystectomy. Korean J Anesthesiol 2015; 68: 455-461. PMID: 26495055.



3. Kim TK, Song HK, Lee JY, Ha NK. Propofol-N2O versus seveflurane-N2O during outpatient knee arthroscopic surgery using the laryngeal mask airway: hemodynamic responses and recovery profiles. Korean J Anesthesiol 2003; 45: 304-309.

4. Brimacombe J. The advantages of the LMA over the tracheal tube or facemask: a meta-analysis. Can J Anaesth 1995; 42: 1017-1023. PMID: 8590490.


5. Kumar G, Stendall C, Mistry R, Gurusamy K, Walker D. A comparison of total intravenous anaesthesia using propofol with sevoflurane or desflurane in ambulatory surgery: systematic review and meta-analysis. Anaesthesia 2014; 69: 1138-1150. PMID: 24847783.


6. Blake DW, Dawson P, Donnan G, Bjorksten A. Propofol induction for laryngeal mask airway insertion: dose requirement and cardiorespiratory effects. Anaesth Intensive Care 1992; 20: 479-483. PMID: 1463177.


7. Kanazawa M, Nitta M, Murata T, Suzuki T. Increased dosage of propofol in anesthesia induction cannot control the patient's responses to insertion of a laryngeal mask airway. Tokai J Exp Clin Med 2006; 31: 35-38. PMID: 21302218.

8. Das A, Majumdar S, Mukherjee A, Mitra T, Kundu R, Hajra BK, et al. i-gelŌäó in ambulatory surgery: a comparison with LMA-ProSealŌäó in paralyzed anaesthetized patients. J Clin Diagn Res 2014; 8: 80-84.

9. i-gel® the supraglottic airway device with non-inflatable cuff [Internet]. Wokingham, UK, Intersurgical Ltd.. updated on 2015 Mar. [date unknown]. Available from: http://www.intersurgical.com/content/files/61953/210806803.
10. Soar J. The i-gel supraglottic airway and resuscitation--some initial thoughts. Resuscitation 2007; 74: 197PMID: 17467873.


11. Almeida G, Costa AC, Machado HS. Supraglottic airway devices: a review in a new era of airway management. J Anesth Clin Res 2016; 7: 647.

12. Dahaba AA. Different conditions that could result in the bispectral index indicating an incorrect hypnotic state. Anesth Analg 2005; 101: 765-773. PMID: 16115989.


13. Bala R, Taxak S, Johar S, Singh R. Comparison of hemodynamic changes associated with insertion of three different supraglottic airway devices (classic LMA vs. PLMA vs. I-Gel) in anaesthetized paralyzed patients. IOSR-JDM 2016; 15: 69-73.
14. Radhika KS, Sripriya R, Ravishankar M, Hemanth Kumar VR, Jaya V, Parthasarathy S. Assessment of suitability of i-gel and laryngeal mask airway-supreme for controlled ventilation in anesthetized paralyzed patients: A prospective randomized trial. Anesth Essays Res 2016; 10: 88-93. PMID: 26957697.



15. Ashay NA, Wasim S, Anil TB. Propofol requirement for insertion of I-gel versus laryngeal mask airway: a comparative dose finding study using Dixon's up-and-down method. J Anaesthesiol Clin Pharmacol 2015; 31: 324-328. PMID: 26330709.



16. Jaap V, Eleske S, Marije R. Intravenous anesthesia. Edited by Miller MDMiller's Anesthesia. 8th ed. : Philadelphia, Churchill Livingstone/Elsevier. 2015, pp 821-863.
17. Kazama T, Morita K, Ikeda T, Kurita T, Sato S. Comparison of predicted induction dose with predetermined physiologic characteristics of patients and with pharmacokinetic models incorporating those characteristics as covariates. Anesthesiology 2003; 98: 299-305. PMID: 12552185.


18. Scanlon P, Carey M, Power M, Kirby F. Patient response to laryngeal mask insertion after induction of anaesthesia with propofol or thiopentone. Can J Anaesth 1993; 40: 816-818. PMID: 8403174.


19. Tanaka M, Nishikawa T. Propofol requirement for insertion of cuffed oropharyngeal airway versus laryngeal mask airway with and without fentanyl: a dose-finding study. Br J Anaesth 2003; 90: 14-20. PMID: 12488372.



20. Bouvet L, Da-Col X, Rimmel├® T, Allaouchiche B, Chassard D, Boselli E. Optimal remifentanil dose for laryngeal mask airway insertion when co-administered with a single standard dose of propofol. Can J Anaesth 2010; 57: 222-229. PMID: 20063135.


21. Kwak HJ, Min SK, Yoo JY, Park KH, Kim JY. The median effective dose of dexmedetomidine for laryngeal mask airway insertion with propofol 2.0 mg/kg. Acta Anaesthesiol Scand 2014; 58: 815-819. PMID: 24961283.


22. Cheam EW, Chui PT. Randomised double-blind comparison of fentanyl, mivacurium or placebo to facilitate laryngeal mask airway insertion. Anaesthesia 2000; 55: 323-326. PMID: 10781116.


23. Kodaka M, Okamoto Y, Handa F, Kawasaki J, Miyao H. Relation between fentanyl dose and predicted EC50 of propofol for laryngeal mask insertion. Br J Anaesth 2004; 92: 238-241. PMID: 14722176.



24. Elbradie S. Neuromuscular efficacy and histamine-release hemodynamic changes produced by rocuronium versus atracurium: a comparative study. J Egypt Natl Canc Inst 2004; 16: 107-113. PMID: 15912151.

25. Mohamed N, Cynthia AL, Claude M. Pharmacology of neuromuscular blocking drugs. Edited by Miller MDMiller's Anesthesia. 8th ed. : Philadelphia, Churchill Livingstone/Elsevier. 2015, pp 958-994.
26. Fujiwara A, Komasawa N, Nishihara I, Miyazaki S, Tatsumi S, Nishimura W, et al. Muscle relaxant effects on insertion efficacy of the laryngeal mask ProSeal(®) in anesthetized patients: a prospective randomized controlled trial. J Anesth 2015; 29: 580-584. PMID: 25667122.


Fig.┬Ā2
Changes in hemodynamic variables during i-gel insertion. Data are presented as the mean ┬▒ SD. (A) systolic blood pressure, (B) diastolic blood pressure, (C) mean blood pressure, and (D) heart rate. *P < 0.05, vs. Group P1.5 (Bonferroni corrected). ŌĆĀP < 0.05, vs. baseline in each group (Bonferroni corrected).
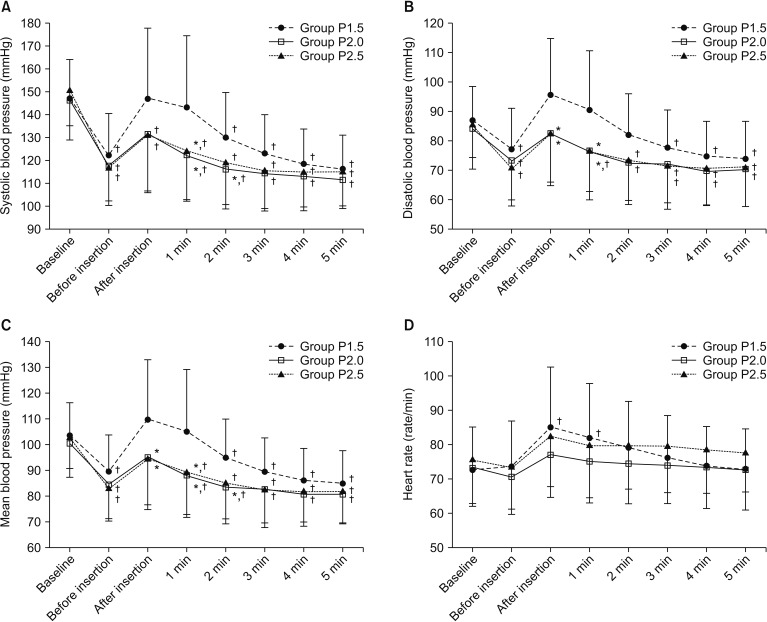
Fig.┬Ā3
Changes in the bispectral index during i-gel insertion. Data are presented as the mean ┬▒ SD. *P < 0.05, vs. Group P1.5 (Bonferroni corrected). ŌĆĀP < 0.05, vs. baseline in each group (Bonferroni corrected).