 |
 |
| Korean J Anesthesiol > Volume 73(2); 2020 > Article |
|
Abstract
Background
The long-term outcomes of patients discharged from the hospital after successful care in intensive care unit (ICU) are not briskly evaluated in Korea. The aim of this study was to assess long-term mortality of patients treated in the ICU and discharged alive from the hospital and to identify predictive factors of mortality.
Methods
In 3,679 adult patients discharged alive from the hospital after ICU care between 2006 and 2011, the 1-year mortality rate (primary outcome measure) was investigated. Various factors were entered into multivariate analysis to identify independent factors of 1-year mortality, including sex, age, severity of illness (APACHE II score), mechanical ventilation, malignancy, readmission, type of admission (emergency, elective surgery, and medical), and diagnostic category (trauma and non-trauma).
Results
The 1-year mortality rate was 13.4%. Risk factors that were associated with 1-year mortality included age (hazard ratio: 1.03 [95% CI, 1.02–1.04], P < 0.001), APACHE II score (1.03 [1.01–1.04], P < 0.001), mechanical ventilation (1.96 [1.60–2.41], P < 0.001), malignancy (2.31 [1.82–2.94], P < 0.001), readmission (1.65 [1.31–2.07], P < 0.001), emergency surgery (1.66 [1.18–2.34], P = 0.003), ICU admission due to medical causes (4.66 [3.68–5.91], P < 0.001), and non-traumatic diagnostic category (6.04 [1.50–24.38], P = 0.012).
Conclusions
The 1-year mortality rate was 13.4%. Old age, high APACHE II score, mechanical ventilation, malignancy, readmission, emergency surgery, ICU admission due to medical causes, and non-traumatic diagnostic category except metabolic/endocrinologic category were associated with 1-year mortality.
According to the 2004 report of the Health Insurance Review and Assessment Service in Korea, 25% of the total medical costs in Korea are spent to treating critically ill patients who hold only 6.4% of the total hospital admission. Furthermore, one-third of the post-intensive care unit (ICU) patients died within 1 month of hospital discharge [1].
For critically ill patients in Korea, in-ICU or in-hospital mortality is still the outcome of greatest interest although it is only a short-term goal. Mortality after discharge is also an important consideration as a long-term goal and as a measurement of the effectiveness of intensive care [2,3]. A number of patients treated in an ICU have a difficult time returning to their lives after hospitalization and the health of some patients continued to decline following discharge [4-6]. The studies on long-term outcomes of ICU survivors are rare in Korea [7,8]. The long-term outcomes are much more difficult to follow-up and certain predictors for mortality after ICU discharge (e.g., quality of life and psychological problems) are not easily measurable. However, determining measurable risk factors for post-treatment mortality may allow for a better allocation of health care resources after discharge and for more efficient targeting of follow-up care.
The aims of this study were to evaluate long-term mortality of patients discharged from the hospital after successful ICU care and to identify predictors of mortality in these patients.
This single-center retrospective study was performed in the general ICU of an 805-bed university-affiliated hospital in Korea and was approved by the Institutional Review Board and Hospital Research Ethics Committee (November 12, 2012, protocol number 3-2012-0209). The ICU has 23 beds and admits about 965 patients per year. As a tertiary center, this general ICU treats almost all categories of critically ill patients, except neonates, open-heart, or neurosurgical patients. Patients who were admitted to the ICU between March 1, 2006, and November 30, 2011, were screened for the study. Among these patients, we selected those who had been discharged alive from the hospital and who were 20 years of age or older (Fig. 1). The following variables were collected using electronic medical records: sex, age, severity of illness at admission (Acute Physiology and Chronic Health Evaluation [APACHE] II score), mechanical ventilation, malignancy, readmission, ICU and hospital length of stay (LOS), type of admission (emergency, elective surgery, and medical), and diagnostic category (trauma and non-trauma such as cardiovascular, respiratory, gastrointestinal/hepatic, renal, neurologic, metabolic/endocrinologic, hematologic, septic shock, and transplantation). The severity of illness was estimated using the APACHE II score and recorded within 24 h of admission to the ICU. The types of admission were assigned at the time of each patient’s admission and recorded within 12 h of ICU admission.
The starting point of long-term survival was defined as the date of discharge from hospital in order to determine the influence of ICU care for discharged patients after a full or partial recovery. Survival time was measured from the day of hospital discharge to December 1, 2012, using the National Health Insurance Service database. All patients were followed up for a minimum of 12 months and a maximum of 76 months. If patients were readmitted to the ICU during this period, only the data from their first admission were considered.
The demographic and clinical characteristics of patients were analyzed using the chi-squared test, independent t-tests, and the Mann-Whitney test. Continuous variables were expressed as mean ± standard deviation (SD). The ICU and hospital LOS, and APACHE II score did not follow a normal distribution. These were expressed as a median and interquartile range (IQR). The survival curve of total post-ICU patients was assessed using the Kaplan-Meier analysis. Univariate and multivariate Cox’s proportional analyses were used to determine independent factors of 1-year mortality (primary outcome measure) and 5-year mortality (secondary outcome measure). Statistically significant variables (P < 0.05) on univariate analysis were included in a stepwise multivariate Cox’s proportional analysis. Statistical significance was defined as a P-value of less than 0.05. Data were analyzed using IBM SPSS Statistics 20 (IBM Corp., USA) and SAS ver. 9.2 (SAS Institute Inc., USA).
A total of 5,351 patients were admitted to the general ICU. Of these patients, 172 patients (younger than 20 years) and 673 patents (ICU readmission) were excluded from this study. A further 827 patients were excluded due to death in the hospital or a terminal prognosis. A total of 3,679 patients were finally included (Fig. 1). The demographic details and clinical characteristics of the participants are shown in Table 1. There were significant differences between post-ICU survivors and non-survivors based on age, APACHE II score, ICU LOS, hospital LOS, mechanical ventilation, malignancy, readmission, type of admission, and diagnostic category (P < 0.001).
The 1-year mortality rate was 13.4%, and a multivariate Cox’s proportional analysis showed that age (hazard ratio [HR] : 1.03 [95% CI, 1.02–1.04], P < 0.001), APACHE II score (1.03 [1.01–1.04], P < 0.001), mechanical ventilation (1.96 [1.60–2.41], P < 0.001), malignancy (2.31 [1.82–2.94], P < 0.001), readmission (1.65 [1.31–2.07], P < 0.001), emergency surgery (1.66 [1.18–2.34], P = 0.003), ICU admission due to medical causes (4.66 [3.68–5.91], P < 0.001), and non-traumatic diagnostic category (6.04 [1.50– 24.38], P = 0.012) were independently associated with mortality (Table 2, Fig. 2).
As the subgroup analysis, we reviewed 831 patients admitted to ICU between 2006 and 2007 for the 5-year follow-up. The 5-year mortality rate was 35.5%, and age (1.04 [1.03–1.05], P < 0.001), mechanical ventilation (1.64 [1.23–2.17], P = 0.001), malignancy (2.02 [1.45–2.81], P < 0.001), readmission (1.56 [1.16–2.11], P = 0.004), and ICU admission due to medical causes (2.07 [1.50–2.86], P < 0.001) were independently associated with mortality (Table 3).
The observed 1- and 5-year mortality rates after hospital discharge in post-ICU patients were 13.4% and 35.5% respectively. The risk factors for 1-year mortality were age, APACHE II score, mechanical ventilation, malignancy, readmission, emergency surgery, ICU admission due to medical causes, and diagnostic category. The risk factors for 5-year mortality were age, mechanical ventilation, malignancy, readmission, ICU admission due to medical causes.
The predictors of long-term mortality have been previously reported [9,10]. In previous studies, the median value of 1-year mortality after critical care treatment in general ICU was 24% (5.4%–44%) [10-18]. These 1-year mortality rates were varied and the median mortality rate higher than our result (24% vs. 13.4%). One study reported 1-year mortality lower than our data (5.4% vs. 13.4%). In this Australian study [10], the starting point of follow-up was hospital discharge but they included patients who were 16 years and older. This difference can be attributed to the fact that some studies [10-18] started tracking mortality after ICU admission or discharge, while our study excluded deaths in the hospital when estimating mortality. The starting point of long-term follow-up is important when assessing mortality. Ranzani et al. [19] reported that if the starting point is changed “ICU admission” to “hospital discharge,” the mean difference is 25% and mean reduction is 54%. Using ICU admission as the starting point for follow-up includes in-ICU mortality. However, when hospital discharge is used as the starting point, the data included patients who are similar to patients with no ICU treatment and had better previous healthy condition. The good medical condition and status performance will influence the post-ICU survival (survivor bias) [19]. For intervention trials, the homogeneity of data should be needed. Ranzani et al. [19] recommended the ICU admission for the starting point because the long-term mortality is not limited to post-discharge mortality and ICU-acquired disease (e.g., ventilator-associated pneumonia, ICU-acquired myopathy, or delirium) should be included in mortality after critical care.
The major predictors of 1-year survival of patients discharged from the ICU were age, severity of disease (APACHE II score), mechanical ventilation, malignancy, readmission, type of admission, and diagnostic category. Many studies [9,10,13,20] have analyzed long-term mortality after ICU treatment, and the most common and most important factors that were associated with survival included age and severity of illness at the time of admission.
The age at ICU admission also appears to affect long-term survival, although age itself influences the death rate [10,20,21]. This factor was a strong predictor of the long-term survival of ICU patients (HR 1.03, P < 0.001).
The mortality rate for ICU patients after hospital discharge increased with the severity of illness. Any comorbidity before ICU admission, whether it was related to ICU admission or not, and the onset of malignancy also affected long-term survival rates [10,22]. Christiansen et al. [23] found that a high pre-admission morbidity level was related to short-term (30-day) and long-term (3-year) mortality rates. They also presented that morbidity had a greater impact on the mortality of ICU patients than that of a general population cohort. Thus, a chronic disease unrelated to the cause of ICU admission could affect the severity of illness and increase long-term mortality even after the acute illness is treated.
We also found various mortality rates and HR between diagnostic categories. Similar to a previous study [11,16], the trauma group had the lowest HR, while the transplantation group had the highest HR. However, our study is not clearly comparable with others [6,10,11,13,16,24-26], as the diagnostic categories were not divided into uniform criteria. We suggest that subdivision of diagnostic categories or homogeneity of cases will be helpful for accurate analysis in future studies.
Long-term survival was also dependent on the type of admission. In this study, long-term survival was the lowest among patients who were admitted for a medical problem. Many studies [9,25-28] have shown various relationships between the type of admission and the long-term prognosis of patients after discharge. Medical patients are more likely to have pre-existing chronic diseases, which may be associated with mortality. Our result showed the 1-year mortality rates of emergency surgical patients were higher than that of elective surgical patients (Fig. 2). In the elective surgical group, included the patients admitted to the ICU for monitoring were included. The APACHE II score of all emergency surgical patients was statistically higher than that of elective surgical patients (emergency: 14.5 ± 7.7, elective: 12.3 ± 6.6, P < 0.001). This difference in severity of illness between the 2 groups might affect the mortality risk at 1 year after hospital discharge.
The 5-year mortality rate in our study was 35.5%, and this rate was similar to previous studies (29.4%–47.9%) [11,13,20,29-31]. Several other studies [10,11,13,20,25] have reported that the highest mortality rate was seen in the first year after ICU treatment, especially in the first 3 months. Meynaar et al. [20] found that the mortality rate in post-ICU patients decreased from 14.6% (first year) to 4.3% (sixth year), and the highest mortality rate was seen in the first 3 months (5.5%). In Western Australia [10], the mortality rate of hospital survivors was highest in the first 12 months (1 yr: 5.4%. 5 yr: 16.3%, 10 yr: 31.3%, 15 yr: 45.3%). We found that the mortality rate was highest in the first year after discharge, while the mortality rate gradually decreased.
The risk factors of 5-year mortality were age, mechanical ventilation, malignancy, readmission, and medical patients. There is a statistically significant difference in 1-year mortality analysis, but no difference in 5-year mortality analysis. In 2013, Brinkman et al. [9] performed the literature review of long-term mortality and Dutch cohort study; they showed a higher hazard ratio of urgent surgery than elective surgery at 3 months after hospital discharge and the risk decreased at 12 months after discharge. They explained this situation by the fact that patients who underwent urgent surgery have fewer comorbidities. In our study, the mortality risk factors at 5 years might be affected by the comorbidities (e.g., the percentage of patients with malignancy among emergency surgery group was 10.4% and elective surgery group was 55.3%). Furthermore, we excluded many patients who underwent cardiovascular surgery (e.g. coronary bypass, valve replacement, or aorta graft surgery) because they were admitted to a cardiac critical care unit instead of the general ICU. Neurosurgical patients, who were diagnosed with a brain tumor or hemorrhage and had frequent emergency surgery, were also excluded from this study in the same manner.
There are neither guidelines nor recommendations about management for improving long-term outcome while the studies about post-intensive care syndrome have been actively conducted. It is important to find the correctable factors for improving long-term mortality of post-ICU patients. The predictors in our study are difficult to modify by any interventions, such as age, severity of illness, malignancy, type of admission, or diagnosis, but the mechanical ventilation and readmission are correctable predictors. In the report from a stakeholder’ conference [32], they recommended some potentially available factors (e.g., pulmonary function, ICU-acquired weakness, cognitive impairment, depression, or posttraumatic stress disorder). Appropriate management of these correctable factors can be helpful in improving long-term outcome of post-ICU patients.
Even though this study was conducted with a large sample size in Korea, the analysis was still limited by several factors. First, data for chronic illnesses before ICU admission and cardiac arrest were not collected; therefore, the effects of these factors on ICU admission and mortality after discharge could not be investigated. Second, the ICU patient population in this study did not include patients undergoing open-heart or neurological surgeries, yet the number of patients presenting with neuromuscular complaints was particularly high. Third, we did not review the interventions performed in the ICU, such as renal replacement therapy or extracorporeal membrane oxygenation. Fourth, we did not evaluate the quality of life that has been widely reported as the important factors of long-term mortality, and we had not the data about the cause of out-hospital death. We are not able to know whether the cause of death is patient’s comorbidity or complication of critical care. Fifth, the readmission rate (10.9%) is extremely high. Although readmission related to the severity of illness, it also means that the ICU discharge was inappropriate. Finally, the purpose of this study was to investigate the factors affecting post-discharge mortality in ICU patients, but there is a limitation that we evaluate only factors known to be associated with hospitals or short-term mortality.
In conclusion, the 1-year mortality rate after hospital discharge in post-ICU patients was 13.4%. Risk factors that were associated with 1-year mortality included old age, high APACHE II score, mechanical ventilation, malignancy, readmission, emergency surgery, ICU admission due to medical causes, and non-traumatic diagnostic categories except metabolic/endocrinologic category.
NOTES
Author Contributions
Se Hee Na (Formal analysis; Software; Writing – original draft; Writing – review & editing)
Cheung Soo Shin (Methodology; Supervision)
Kim Gwan Ho (Data curation; Investigation)
Jae Hoon Kim (Data curation)
Jong Seok Lee (Conceptualization; Writing – original draft; Writing – review & editing)
Fig. 1.
Flow diagram of patient screening for this study. The schematic shows the inclusion criteria for patients admitted to the ICU from 2006 to 2011. DNR: do not resuscitation, ICU: intensive care unit.
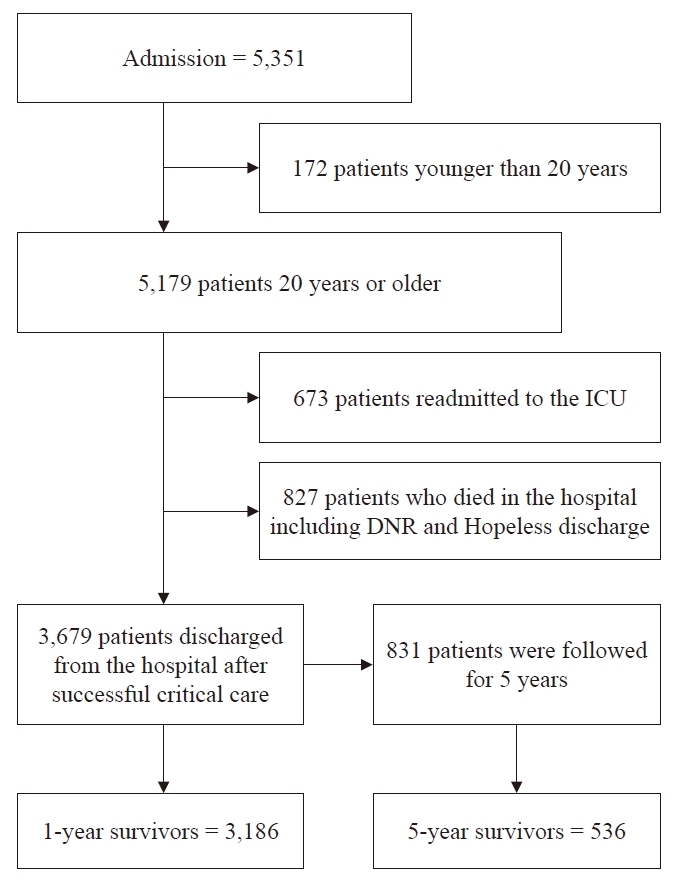
Fig. 2.
Cox’s regression analysis curves showing 1-year mortality after hospital discharge of the total post-ICU patients and subgroups based on the type of admission. ICU: intensive care unit. *Emergency surgical patients have a higher mortality compared with elective surgical patients (P = 0.003). †Medical patients have a higher mortality compared with elective surgical patients (P < 0.001).
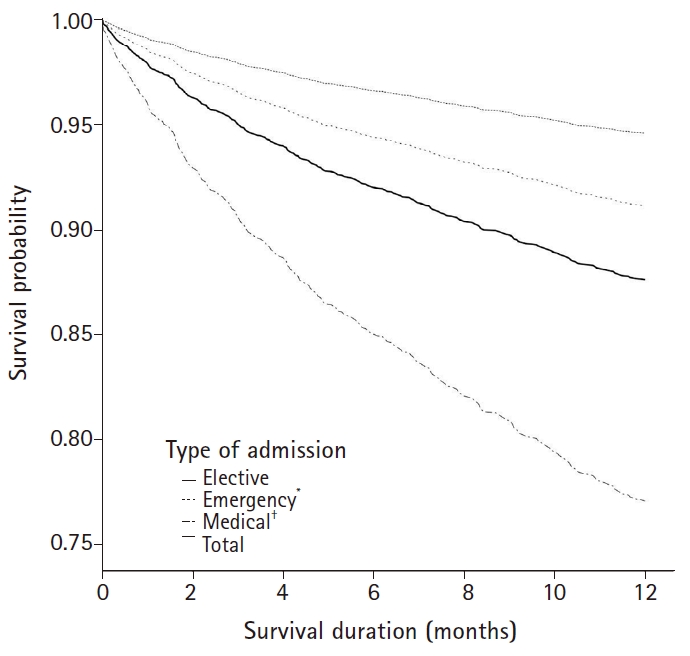
Table 1.
Demographic and Clinical Characteristics of Patients Admitted from March 1, 2006, to November 30, 2011
Table 2.
Univariate and Multivariate Cox’s Proportional Analysis of Variables Associated with 1-year Mortality
Table 3.
Univariate and Multivariate Cox’s Proportional Analysis of Variables Associated with 5-year Mortality
References
1. Health Insurance Review Agency. ICU benefits appropriateness evaluation report. HIRA e-Book [Internet]. Seoul: 2004 Dec [cited 2018 Sep 17]. Available from: https://www.hira.or.kr/sViewer/index.do?ebookSn=418
2. Angus DC, Carlet J, Brussels Roundtable P. Surviving intensive care: a report from the 2002 Brussels roundtable. Intensive Care Med 2003; 29: 368-77.



3. Dowdy DW, Needham DM, Mendez-Tellez PA, Herridge MS, Pronovost PJ. Studying outcomes of intensive care unit survivors: the role of the cohort study. Intensive Care Med 2005; 31: 914-21.



4. Jackson JC, Pandharipande PP, Girard TD, Brummel NE, Thompson JL, Hughes CG, et al. Depression, post-traumatic stress disorder, and functional disability in survivors of critical illness in the brain-ICU study: a longitudinal cohort study. Lancet Respir Med 2014; 2: 369-79.



5. Norman BC, Jackson JC, Graves JA, Girard TD, Pandharipande PP, Brummel NE, et al. Employment outcomes after critical illness: an analysis of the bringing to light the risk factors and incidence of neuropsychological dysfunction in ICU survivors cohort. Crit Care Med 2016; 44: 2003-9.



6. Eddleston JM, White P, Guthrie E. Survival, morbidity, and quality of life after discharge from intensive care. Crit Care Med 2000; 28: 2293-9.


7. Heo SJ, Kim G, Lee CK, Chung KS, Choi HJ, Sohn J, et al. Prediction of short- and long-term survival for advanced cancer patients after ICU admission. Support Care Cancer 2015; 23: 1647-55.



8. Heo J, Hong Y, Han SS, Kim WJ, Kwon JW, Moon KW, et al. Changes in the characteristics and long-term mortality rates of intensive care unit patients from 2003 to 2010: a nationwide population-based cohort study performed in the Republic of Korea. Acute Crit Care 2018; 33: 135-45.




9. Brinkman S, Bakhshi-Raiez F, Abu-Hanna A, de Jonge E, de Keizer NF. Determinants of mortality after hospital discharge in ICU patients: literature review and Dutch cohort study. Crit Care Med 2013; 41: 1237-51.


10. Williams TA, Dobb GJ, Finn JC, Knuiman MW, Geelhoed E, Lee KY, et al. Determinants of long-term survival after intensive care. Crit Care Med 2008; 36: 1523-30.


11. Wright JC, Plenderleith L, Ridley SA. Long-term survival following intensive care: subgroup analysis and comparison with the general population. Anaesthesia 2003; 58: 637-42.


12. Keenan SP, Dodek P, Chan K, Hogg RS, Craib KJ, Anis AH, et al. Intensive care unit admission has minimal impact on long-term mortality. Crit Care Med 2002; 30: 501-7.


13. Niskanen M, Kari A, Halonen P. Five-year survival after intensive care—comparison of 12,180 patients with the general population. Finnish ICU Study Group. Crit Care Med 1996; 24: 1962-7.


14. Laupland KB, Zygun DA, Doig CJ, Bagshaw SM, Svenson LW, Fick GH. One-year mortality of bloodstream infection-associated sepsis and septic shock among patients presenting to a regional critical care system. Intensive Care Med 2005; 31: 213-9.



15. Rockwood K, Noseworthy TW, Gibney RT, Konopad E, Shustack A, Stollery D, et al. One-year outcome of elderly and young patients admitted to intensive care units. Crit Care Med 1993; 21: 687-91.


16. Ridley S, Jackson R, Findlay J, Wallace P. Long term survival after intensive care. BMJ 1990; 301: 1127-30.



17. Engoren M, Arslanian-Engoren C. Long-term survival in the intensive care unit after erythrocyte blood transfusion. Am J Crit Care 2009; 18: 124-31.


18. Nilsson G, Astermark J, Lethagen S, Vernersson E, Berntorp E. Protein C levels can be forecasted by global haemostatic tests in critically ill patients and predict long-term survival. Thromb Res 2005; 116: 15-24.


19. Ranzani OT, Zampieri FG, Park M, Salluh JIF. Long-term mortality after critical care: what is the starting point? Critical Care 2013; 17: 191.



20. Meynaar IA, Van Den Boogaard M, Tangkau PL, Dawson L, Sleeswijk Visser S, Bakker J. Long-term survival after ICU treatment. Minerva Anestesiol 2012; 78: 1324-32.

21. Sacanella E, Perez-Castejon JM, Nicolas JM, Masanes F, Navarro M, Castro P, et al. Mortality in healthy elderly patients after ICU admission. Intensive Care Med 2009; 35: 550-5.



22. Schneider CP, Fertmann J, Geiger S, Wolf H, Biermaier H, Hofner B, et al. Long-term survival after surgical critical illness: the impact of prolonged preceding organ support therapy. Ann Surg 2010; 251: 1145-53.


23. Christiansen CF, Christensen S, Johansen MB, Larsen KM, Tønnesen E, Sørensen HT. The impact of pre-admission morbidity level on 3-year mortality after intensive care: a Danish cohort study. Acta Anaesthesiol Scand 2011; 55: 962-70.


24. Chelluri L, Pinsky MR, Donahoe MP, Grenvik A. Long-term outcome of critically ill elderly patients requiring intensive care. JAMA 1993; 269: 3119-23.


25. Flaatten H, Kvale R. Survival and quality of life 12 years after ICU. A comparison with the general Norwegian population. Intensive Care Med 2001; 27: 1005-11.



26. Brinkman S, de Jonge E, Abu-Hanna A, Arbous MS, de Lange DW, de Keizer NF. Mortality after hospital discharge in ICU patients. Crit Care Med 2013; 41: 1229-36.


27. Parno JR, Teres D, Lemeshow S, Brown RB, Avrunin JS. Two-year outcome of adult intensive care patients. Med Care 1984; 22: 167-76.


28. Laupland KB, Kirkpatrick AW, Kortbeek JB, Zuege DJ. Long-term mortality outcome associated with prolonged admission to the ICU. Chest 2006; 129: 954-9.


29. Luangasanatip N, Hongsuwan M, Lubell Y, Limmathurotsakul D, Teparrukkul P, Chaowarat S, et al. Long-term survival after intensive care unit discharge in Thailand: a retrospective study. Crit Care 2013; 17: R219.



30. Cuthbertson BH, Roughton S, Jenkinson D, Maclennan G, Vale L. Quality of life in the five years after intensive care: a cohort study. Crit Care 2010; 14: R6.












