Introduction
An injection of botulinum toxin is commonly used to relieve spasticity and prevent contractures in children with spastic cerebral palsy (CP). Because of the pain associated with the injection and the inability for movement control during the procedure, the maintenance of deep sedation with effective analgesia is important so that the botulinum toxin can be accurately administered in the right muscle. However, because children may easily transit from deep sedation to general anesthesia with an increased risk of cardiorespiratory depression, a repeated and accurate assessment of the sedation level is necessary for the safety of children undergoing procedural sedation.
Clinical sedation scales, such as the University of Michigan Sedation Scale (UMSS) and the Observer’s Assessment of Alertness and Sedation (OAAS) scale, have been used as valid tools to assess the level of sedation in children by observing their response to various stimuli [
1,
2]. However, the intermittence of the assessment, the variance between observers, and the disruptive effect of the stimuli while assessing the level of sedation during a procedure may hinder their utility in clinical practice. The bispectral index (BIS) has been widely used as a continuous, objective, and quantitative assessment tool for evaluating the level of sedation in adults by incorporating various electroencephalogram (EEG) signals into a single dimensionless number ranging from 0 (isoelectric silence) to 100 (full wakefulness) [
3,
4]. A BIS score lower than 40 represents deep hypnosis and a score higher than 80 may be associated with recall. Previous studies have suggested that the BIS is also a valid monitoring tool for assessing the level of sedation in pediatric patients undergoing diagnostic or therapeutic procedures [
5,
6]. Previous studies have reported that the baseline BIS values in children with CP are similar to those in normal children at wakefulness, before premedication [
7,
8], but lower than the same after midazolam premedication [
5,
6]. In addition, children with CP required a lower propofol dose than that required in normal children to obtain the same BIS value during propofol anesthesia [
7]. Therefore, the validity of BIS monitoring for assessing the level of procedural sedation in children with CP has yet to be established.
This prospective observational study aimed to determine whether the BIS is a valid objective tool for differentiating adequate deep sedation from inadequate sedation in spontaneously breathing children with spastic CP. Our hypothesis was that the BIS values would have a strong correlation with the UMSS and the Modified Observer’s Assessment of Alertness and Sedation scale (MOAAS) scores during sedation in children with CP undergoing a botulinum toxin injection.
Materials and Methods
This prospective observational study was approved by the Institutional Review Board (YUH-14-0319-O10) and was registered with ClinicalTrials.gov (NCT02096549) on March 28, 2014. Written informed consent from the participants’ parents and, when appropriate, verbal assent from the participating children were obtained before enrollment in the study. A total of 22 children with spastic CP, with an American Society of Anesthesiologists physical status of 1 and 2, aged between 3 and 18 years, who were scheduled for a botulinum toxin injection under deep sedation, were enrolled. Children were excluded from the study if they had an anticipated difficult airway, unstable cardiac disease, craniofacial defect, allergy to drugs used in this study, and/or history of recent (< 8 weeks) pneumonia, bronchitis, asthma attack, or an upper respiratory infection.
None of the participants received premedication. A 22-gauge intravenous catheter was inserted at a superficial vein in the arm or hand by an expert nurse 1 h before the estimated induction of sedation. Upon arrival into the operating room with one of the parents, the children were placed in a supine position with a soft roll under their shoulders to slightly extend the neck. The children were continuously monitored using electrocardiography, noninvasive blood pressure (NIBP), and pulse oximetry. An age-and head size-appropriate BIS sensor was applied unilaterally to the forehead and was connected to a BIS monitoring system (VISTATM; Aspect Medical System, USA) in accordance with the manufacturer’s instruction. The BIS monitor screen was covered during the procedure to ensure that the anesthesiologist who was responsible for the induction and maintenance of sedation and analgesia was blinded to the BIS value. Supplemental oxygen was administered through a simple face mask at a flow rate of 5 L/min throughout the procedure. All baseline data were collected before the administration of remifentanil.
Before the administration of propofol, a remifentanil infusion was started at a rate of 0.05 μg/kg/min without an initial bolus. Five minutes after the start of the remifentanil infusion, the attending anesthesiologist administered propofol (1 mg/kg) premixed with lidocaine (1 mg/kg) over 1 min, followed by a continuous infusion at a rate of 5 mg/kg/h. The propofol infusion rate was increased by 1 mg/kg/h every 1 min, up to 10 mg/kg/h, until an adequate sedation was achieved. The ventilation during sedation was monitored by the respiratory rates per minute and the continuous waveform of expired CO
2 using a sampling line located around a nostril inside the face mask and connected to a side-stream capnography. In case of an oxygen saturation < 93%, end-tidal carbon dioxide concentration > 50 mmHg, apnea (cessation of spontaneous ventilation for 20 s), or a 20% decrease in the heart rate or blood pressure from the baseline values, an intervention was considered with discontinuation of the propofol infusion. Two independent investigators assessed the level of sedation using the UMSS and the MOAAS scales every 1 min during the induction of sedation and every 3 min during the maintenance of sedation (
Table 1). The MOAAS scale includes only the responsiveness component of the original OAAS scale [
9]. These investigators were blinded to the BIS values throughout the study period. Another, third investigator recorded the BIS and electromyography (EMG) values, oxygen saturation, respiratory rate, BP, and heart rate immediately before each assessment of the clinical sedation score. All EMG data, BISs, and signal quality indices (SQIs) were downloaded at 1-minute intervals. The BIS values with a SQI ≥ 50 were accepted for analysis. The BIS values 1 min before and after stimulation at each clinical sedation score were recorded and averaged. Deep sedation was defined based on the clinical sedation scales’ categories, that is, a UMSS score of 3–4 or an MOAAS score of 0–1 [
10,
11]. A sample size of 19 was calculated to provide the anticipated correlation coefficient of 0.6 between the BIS values and the clinical sedation scores with an α-error of 0.05 and a power of 80% using G*Power 3.1.3 [
12]. We enrolled 22 patients, taking into consideration a 15% drop-out rate.
Statistical analysis was performed using SPSS, version 22.0 (SPSS Inc., USA) for Windows. Continuous variables were presented as means ± standard deviation for normally distributed data or medians with an interquartile range for non-normally distributed data after the Kolmogorov-Smirnov test with Lilliefors correction. Categorical variables were presented as numbers with percentages. The BIS values at each UMSS and MOAAS score were compared using one-way analysis of variance (ANOVA), followed by multiple pairwise comparisons with Bonferroni correction. The BIS values were categorized into ranges of < 50, 50–59, 60–69, 70–79, 80–89, and ≥ 90. The kappa coefficient was calculated to determine the agreement between the two independent observers regarding the clinical sedation scores. A kappa ≥ 0.7 was considered a good agreement. The correlation between the paired BIS value categories and clinical sedation scores was determined using the Spearman rank correlation test. Receiver operating characteristic (ROC) curve analysis was performed to determine the sensitivity and specificity of the BIS for predicting deep sedation based on the clinical sedation scores. A cutoff BIS value for detecting deep sedation in children with CP was also calculated. A P value ≤ 0.05 was considered to be statistically significant.
Results
A total of 20 children with spastic CP were included in the final analysis (
Table 2). Two patients were excluded due to a low SQI of the BIS. All children maintained stable blood pressure and heart rate throughout the intervention. The amount of propofol in combination with remifentanil required for the induction of sedation was 1.4 ± 0.4 mg/kg and that required for maintenance of the deep sedation during the botulinum toxin injection was 8.7 ± 4.4 mg/kg/h. Spontaneous breathing was maintained without a respiratory depression (respiratory rate < 10 breaths/min) in all children during the intervention. However, a temporary jaw thrust maneuver and positive pressure ventilation due to a transient desaturation (oxygen saturation < 93%) were required at a UMSS score of 4 and a MOAAS score of 0 in two and one of the patients, respectively.
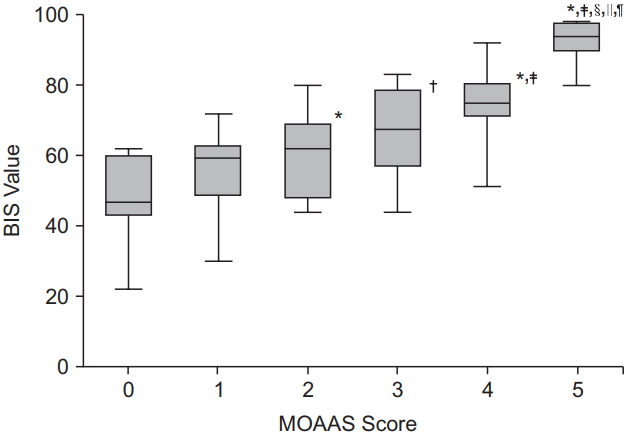
The baseline pre-sedation BIS score before the administration of remifentanil was 93.1 ± 5.1. There was a good agreement between the two independent investigators who assessed the clinical sedation scores (UMSS score: kappa 0.787, P < 0.001; MOAAS score: kappa 0.831, P < 0.001). The relationships between the BIS values and each clinical sedation scale score are shown in
Figs. 1 and
2. The median BIS values significantly changed as the level of sedation increased across both the UMSS and MOAAS scores (P < 0.001). The categorized BIS values significantly correlated with the UMSS (
Fig. 1, r = −0.795, P < 0.001) and MOAAS scores (
Fig. 2, r = 0.815, P < 0.001).
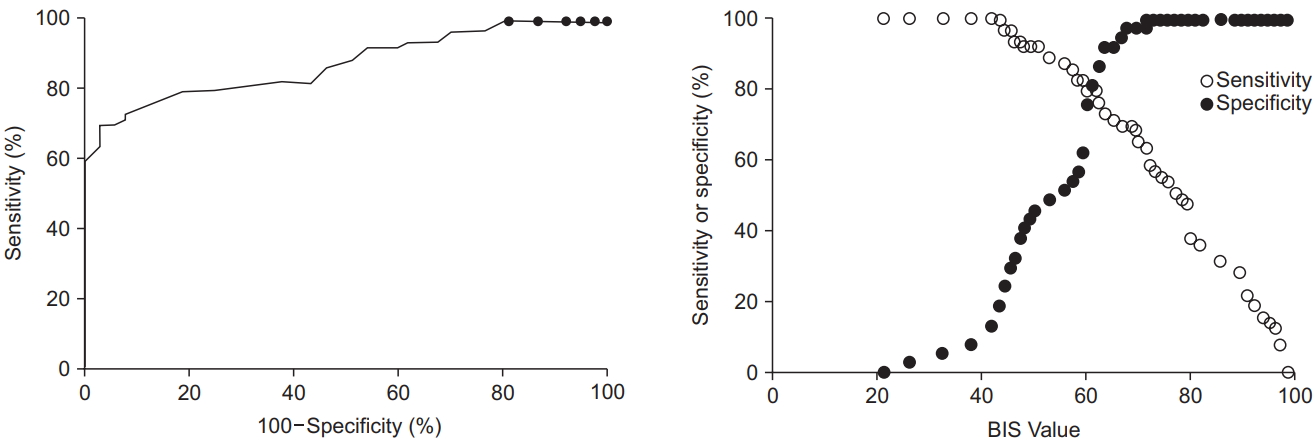
The ROC analysis revealed that the area under the curve was 0.917 for the UMSS score (95% CI 0.858 to 0.969, P < 0.001,
Fig. 3) and 0.874 for the MOAAS score (95% CI 0.808 to 0.940, P < 0.001,
Fig. 4). The cutoff BIS value to detect an adequate deep sedation in children with CP was 61.5 (sensitivity 0.860 and specificity 0.814 based on the UMSS score of 3–4; sensitivity 0.794 and specificity 0.811 based on the MOAAS score of 0–1).
Discussion
In the present study, we demonstrated that continuous BIS monitoring can reliably detect deep sedation in spontaneously breathing children with CP undergoing a botulinum toxin injection. The BIS scores correlated with the clinically observed UMSS and MOAAS scores during propofol sedation. A BIS score of 61.5 was determined as the threshold value that best discriminated between an adequate deep sedation and an inadequate sedation in children with CP undergoing a propofol and remifentanil regimen for invasive procedures.
Several previous studies have reported a relationship between the changes in the EEG waves and the level of sedation. The EEG-derived BIS, rather than the clinical sedation scales, can evaluate the level of sedation objectively without interrupting the procedure, because differentiating a reliable patient’s response to significant physical stimuli is difficult in paralyzed or deeply sedated patients. Several studies conducted in healthy children have reported a good correlation between the BIS and validated observational scales, such as the Ramsay scale and UMSS, during invasive and noninvasive procedures [
10,
12–
14].
Because the BIS is developed on the basis of studies conducted in patients with a normal EEG, its validity and reliability could be questionable in children with CP, who have central nervous system abnormalities. Previous studies have reported that the baseline BIS values in children with CP are similar to those in normal children at wakefulness, before premedication [
7,
8], but lower than the same after midazolam premedication [
5,
6]. During sevoflurane anesthesia, children with CP who had quadriplegia and mental retardation required lower anesthetic doses than those required in normal children, but exhibited a pattern in the change of the BIS scores similar to that observed in normal children [
5]. Kim et al. [
15] also found no difference in the BIS, response entropy, and state entropy between normal and children with CP during sevoflurane anesthesia induction. Saricaoglu et al. [
7] found that the BIS values were comparable between normal and noncommunicative/nonverbal children with CP at baseline and after propofol administration. These results suggest that continuous BIS monitoring can be a useful tool for titrating the anesthetic dosage to obtain an appropriate level of anesthesia and, consequently, prevent excessive anesthesia, which increases the risk of adverse events in children with CP who have a greater sensitivity to anesthetic drugs.
However, the usefulness of BIS monitoring in deeply sedated children with CP during procedural sedation has not been established. In normal children, the BIS scores have been reported to significantly correlate with the clinical observational scoring scales, such as the UMSS and OAAS, during procedural sedation [
10,
13,
16]. In the present study, the BIS scores correlated well with their paired UMSS (r = −0.795) and MOAAS (r = 0.815) scores in children with CP during procedural sedation for an invasive procedure. Our findings suggested that BIS monitoring can be useful for assessing the level of sedation in neurologically abnormal children, such as children with CP, during procedural sedation, similarly as in normal children.
It is important to understand the influence of commonly used sedative and analgesic regimens on the relationship between the BIS scores and the observational sedation scores. There was a moderate-to-high correlation between the BIS and the UMSS scores when using chloral hydrate, pentobarbital, and midazolam. In contrast, a poor correlation between them was reported when using ketamine or opioids during procedural sedation in children [
17]. It was also reported that remifentanil, even at large doses, did not induce modifications in the BIS obtained under a constant level of propofol infusion [
18]. In our present study, we used only propofol and remifentanil in children with CP undergoing a botulinum toxin injection to eliminate the variability in the sedative and analgesic regimen and the severity of the procedure. We found that the cutoff BIS value for deep sedation in children with CP undergoing an invasive procedure was 61.5, based on the observation of the patients’ responsiveness to stimuli using the UMSS and MOAAS scales. These results indicated that the BIS value for deep sedation is comparable to the level of general anesthesia in children with CP who received propofol for procedural sedation. These low BIS values for deep sedation in children with CP are consistent with the previously reported cutoff BIS value of 55.6 in normal children deeply sedated with propofol [
17]. Previous studies that used propofol titration during deep sedation for painful procedures also reported mean BIS scores of approximately 62 in neurologically intact children [
12,
19]. Taken together, these findings indicate that the titration of propofol based on a continuous and objective monitoring of the sedation level is imperative in both normal and children with CP to prevent drifting into general anesthesia during deep sedation, because it is hard to detect the response of patients to various stimuli using clinical sedation scales.
The most frequent adverse event in this study was transient desaturation, which coincided with unresponsiveness to painful stimuli (a UMSS score of 4 and a MOAAS score of 0). Although propofol alone can produce an effective sedation, larger doses may be required to suppress movement during painful procedures. The combination of sedative and systemic analgesic agents generally provides an optimal control of amnesia, analgesia, and immobility at a low dose of each drug. We carefully titrated the dose of propofol in addition to a continuous infusion of a low-dose remifentanil (0.05 μg/kg/min) without an initial bolus to achieve a desirable level of sedation while maintaining spontaneous ventilation in our patients. Although the analgesic concentration of remifentanil used in this study was far less than the effect-site concentration that would induce respiratory depression [
20], the propofol-remifentanil interaction can result in strikingly synergic respiratory depression, even at low doses [
21]. The occurrence of hypoxemia in very deeply sedated patients, similar to the monitoring of the respiratory function, indicates that the titration of the propofol dose with a continuous and objective monitoring of the sedation level using the BIS may be helpful to prevent an oversedation-associated respiratory depression during deep sedation.
Some limitations of this study have to be addressed. First, the clinical sedation scales used in this study did not clearly differentiate between deep sedation and general anesthesia. In accordance with previous reports [
10,
17], the definition of deep sedation used in this study included a purposeful response and unresponsiveness to repeated or painful stimulation, which is consistent with general anesthesia. In addition, although both UMSS and OAAS have been widely used and validated for measuring the level of sedation in children [
1,
9], the injection of botulinum toxin itself may be a standardized and valid stimulus for assessing the BIS target during deep sedation in our patients. Second, the BIS value may differ significantly before and after stimulation. The measurement of BIS values after stimulation can enable the prediction of a possible unwanted under- or oversedation. Thus, the combined use of BIS values before and after stimulation could raise its clinical utility as an early screening tool for an undesirable level of sedation during a procedure. Third, although we investigated a relatively homogenous cohort of 20 children with spastic CP, the influence of the variation in the individual neurologic deficits and the age-related differences in brain maturation and synapse formation throughout childhood on the BIS monitoring cannot be excluded [
22]. In addition, we could not eliminate the variability in the BIS values in patients with seizure receiving anticonvulsant medication. Prospective studies with a larger sample are needed to develop optimal sedation guidelines for children with CP according to the severity, development, clinical manifestation, and associated disorders.
In conclusion, the present study indicated that the BIS value has a good correlation with the clinical sedation scales, such as the UMSS and MOAAS, during deep sedation in spontaneously breathing children with CP undergoing an injection of botulinum toxin. A BIS score of 61.5 is the threshold value of deep sedation in children with CP when receiving propofol and remifentanil regimen. Therefore, continuous and objective BIS monitoring can be used as a valid tool for inducing and maintaining deep sedation in these patients.