The analgesic effect of ultrasound-guided transverse abdominis plane block after laparoscopic totally extraperitoneal hernia repair
Article information
Abstract
Background
The ultrasound-guided transverse abdominis plane block (TAPB) reduces postoperative pain after laparoscopic abdominal surgery. But, its effect post laparoscopic totally extraperitoneal hernia repair (TEP) is not clear. In this study, we evaluated the analgesic effect of ultrasound-guided TAPB in TEP.
Methods
In this prospective, randomized study, forty adult patients (ASA I-II) scheduled for a TEP under general anesthesia were studied. In the TAPB group (n = 20), an ultrasound-guided bilateral TAPB was performed with 0.375% ropivacaine 15 ml on each side after the induction of general anesthesia. The control group (n = 20) did not have TAPB performed. Fentanyl 50 µg was repeatedly injected as per the patient's request in the recovery room. Pain scores at rest and on coughing were assessed postoperatively in the recovery room (20 min, at discharge) and at 4, 8, and 24 hours after surgery.
Results
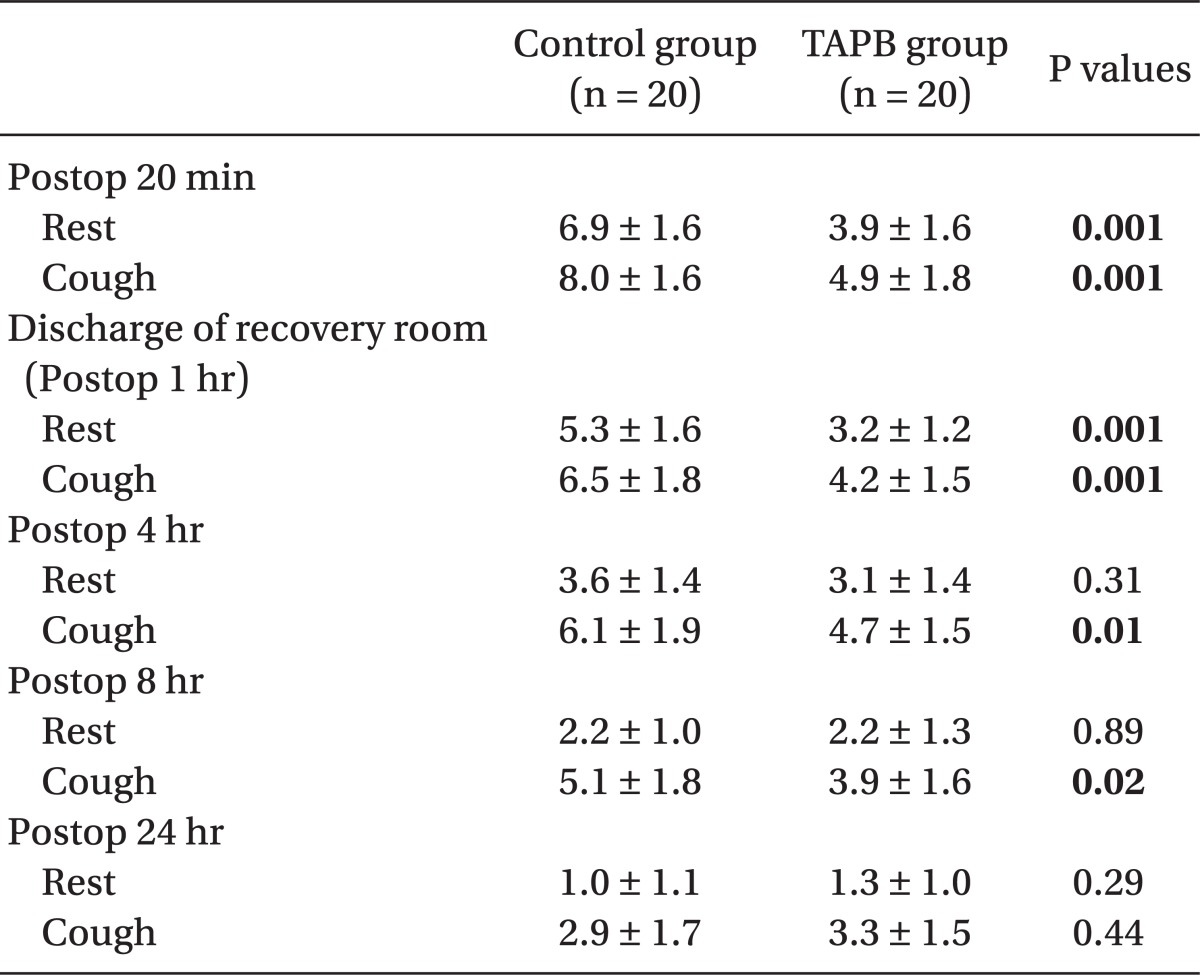
In the recovery room, pain scores (numeric rating scale, 0-10) at postoperative 20 min were lower in the TAPB group (3.9 ± 1.6, 4.9 ± 1.8) than the control group (6.9 ± 1.6, 8.0 ± 1.6) at rest and on coughing. Also, pain scores upon discharge from the recovery room were lower in the TAPB group (3.2 ± 1.2, 4.2 ± 1.5) than the control group (5.3 ± 1.6, 6.5 ± 1.8) at rest and on coughing.
Conclusions
The ultrasound-guided TAPB in patients that had undergone TEP reduced postoperative pain scores and the fentanyl requirement in the recovery room. Also, pain scores on coughing were reduced until postoperative 8 hours.
Introduction
Inguinal hernia repair is one of the most common surgeries performed by surgeons. Open and laparoscopic herniorraphy are both used, but since the evolution of surgical tools and new surgical skills in the 1990's, laparoscopic procedure have become more prevalent. There are two laparoscopic methods: laparoscopic transabdominal preperitoneal hernia repair (TAPP) and laparoscopic totally extraperitoneal hernia repair (TEP). The choice for the surgical method depends on the surgeon's skills, preference, and the patient's condition. The prevalence of laparoscopies is due to the advantage of fewer complications, decrease in hospital length of stay, speed of recovery, fast return to activities of daily living and work [1,2].
Generally laparoscopic surgeries are known to be relatively less painful, but still require pain management. Many attempt at post-operative pain control following a laparoscopic cholecystectomy exist. The infiltration of local anesthetic at the trocar insertion site, into the intraperitoneal space or blood vessel by direct local anesthetic administration are effective for postoperative pain reduction [3-5]. Also the ultrasound-guided transverse abdominis plane block (TAPB) is reported to be safe to perform and to reduce perioperative and postoperative pain and reduce the dosage of opioid analgesics [6,7].
In our pilot study patients in the recovery room following a TEP had a mean pain score of 7.0 ± 1.7 on the numeric rating scale (NRS), and one patient needed a great dosage of analgesics (up to fentanyl 200 µg). Thus post-operative pain control is deemed necessary. Since TEP is performed in a localized site in the extraperitoneal lower abdomen and somatic pain is considered to be greater than visceral pain, TAPB is considered to greatly help in postoperative pain control. There are very few studies focused on pain and TAPB in TEP patients. So the authors performed the present study to find the analgesic effects of ultrasound-guided TAPB's analgesic effects on TEP patients.
Materials and Methods
The present study had forty TEP patient participants (ASA I-II) aged 20 and older. We received permission from the hospital ethics committee. We explained the purpose and method of the study then received their written consent before starting the study. Patients were excluded from the study if they had a past history of hernia repair or complained of severe pain at the surgical site before the operation, or who had little understanding of the pain score.
We randomly divided patients into the TAPB group (n = 20) which had TAPB and the control group (n = 20), which did not. We gave an intramuscular injection of glycopyrrolate 0.2 mg to all patients before surgery as premedication. Remifentanil 1 µg/kg, lidocaine 40 mg, propofol 1-2 mg/kg, rocuronium 0.6 mg/kg iv. was used for anesthesia induction before endotracheal intubation. We used oxygen 2 L/min, nitrous oxide 2 L/min, desflurane 4 vol% and continuously infused remifentanil for anesthesia maintenance. During surgery, we monitored the patient with NIBP, ECG, arterial oxygen saturation, capnography, and bispectral index (BIS).
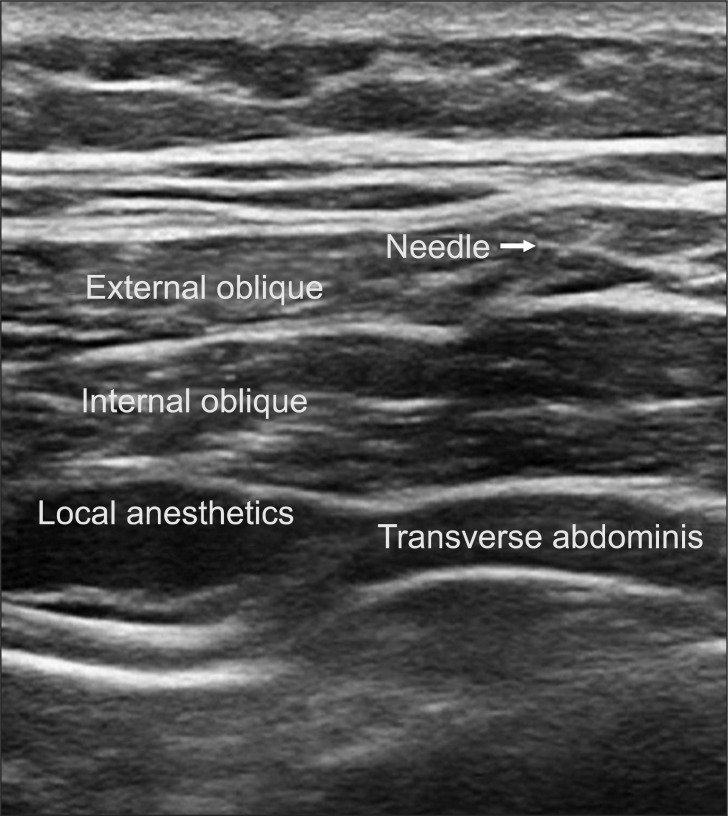
We performed TAPB after anesthesia induction using the method by Hebbard et al. [8]. With an ultrasound guide, we placed a probe immediately above the iliac crest vertical to the mid-axillary line, inserted a 22 G blunt needle, and injected 0.375% ropivacaine 15 ml into the transverse abdominis plane (TAP). The opposite side was performed in the same manner (Fig. 1). Also, during surgery we maintained BIS at 40-60 and and kept mean arterial pressure at 60-100 mmHg by adjusting the remifentanil dosage. When the skin was sutured, we stopped the continuous infusion of remifentanil and recorded the dosages used.
The surgery was performed using the traditional TEP method (Fig. 2). Carbon dioxide infusion pressure was set at 12 mmHg, a mesh was placed and secured with a tacker. All surgeries were performed by the same surgeon. After surgery, glycopyrrolate and pyridostigmine used to reverse the muscle relaxants.
Upon twenty minutes after arriving in the recovery room, pain scores were taken at rest and on coughing using NRS (0: no pain, 10: greatest pain). Pain was blindly assessed and recorded before the patient was discharged from the recovery room (post-surgery 1 hour), post-surgery 4 hours, 8 hours, and 24 hours by an anesthesiology resident who was blinded to the present study. Also, a sleep disturbance 24 hours after surgery was recorded. If the patient complained of pain in the recovery room, the pain score was recorded and fentanyl 50 µg was administered. Ten minutes following, pain was reassessed. If it was not controlled or the patient was not satisfied, fentanyl 50 µg readministered until there were no side-effects of fentanyl. Afterwards, on the patient ward, tramadol hydrochloride 50 mg iv was administered twice in an 8-hr interval. If the patient requested additional analgesics, tramadol hydrochloride 50 mg was administered.
In the pilot study NRS in the recovery room for the control group was 7 with a standard deviation of 1.7, so a NRS below 5 for the TAPB group was considered clinically significant. By calculating α = 0.05 and power = 0.9, each group was supposed to have 17 patients as for sample size. Statistical analysis was done using SPSS (version 14.0, SPSS Inc., Chicago, IL, USA). The Student t-test was used for continuous variables and the chi-square test and Fisher's exact test were used for categorical variables. Pain scores were shown as mean ± standard deviation. P value below 0.05 was considered statistically significant.
Results
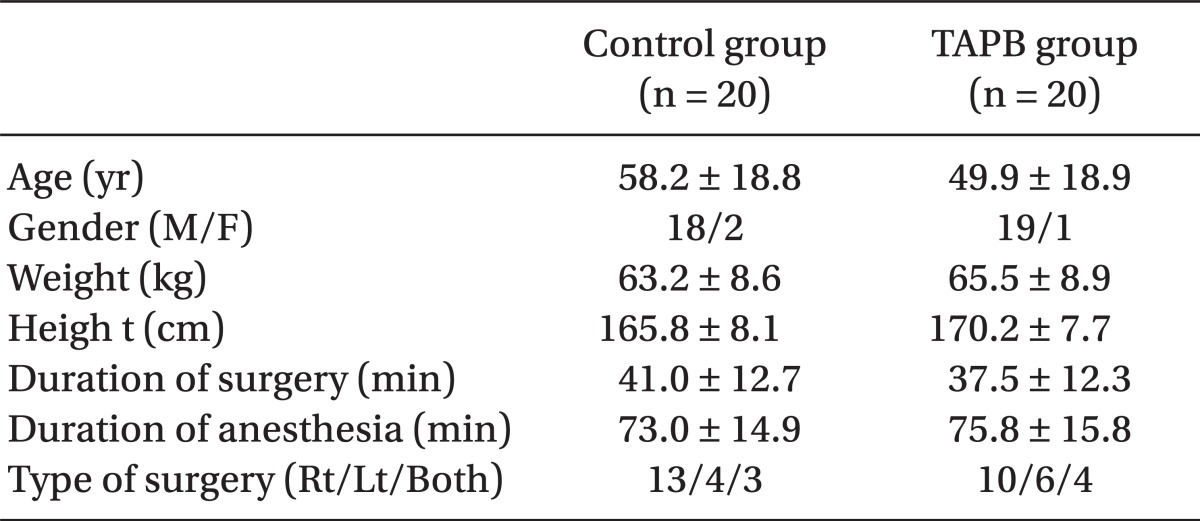
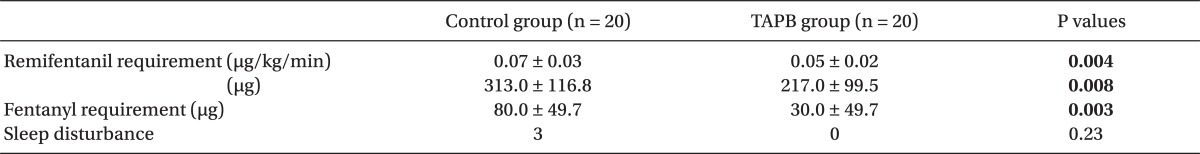
There were no differences between the two groups in age, gender, height, weight, operative time, anesthesia time, or surgical site (Table 1). Post-operative pain in the TAPB group compared to the control group was significantly low at 20 min after surgery and at discharge from the recovery room. 4 hours and 8 hours following surgery only pain on coughing was significantly low in the TAPB group (Table 2). After 24 hours there was no difference between levels of pain at rest and when coughing. The mean dosage of remifentanil administered during surgery and the mean dosage of fentanyl administered in the recovery room was significantly lower in the TAPB group (Table 3). Also, the number of patients who wanted analgesics in the recovery room was significantly lower in the TAPB (8 patients from the TAPB group and 17 patients from the control group; P = 0.008). Only 2 patients from the control group wanted extra analgesics on the ward, so they each were administered tramadol hydrochloride 50 mg iv once. There was no statistical significance between the two groups in sleep disturbance.
Discussion
Laparoscopic surgeries are known to involve less pain compared to open surgeries. But there are many patients who complain of severe postoperative pain, and there are cases where this can develop into chronic pain. So there are studies underway focused on methods to control pain. Invasive stimulation due to surgery can cause central sensitization which can lead to chronic pain. Preemptive analgesia [9] has been introduced to block this, and studies have been performed using it even in laparoscopic surgeries. Also pain characteristics have been categorized in an attempt to effectively control different types of pain.
Because of viscera-somatic convergence, it is difficult to clinically differentiate somatic and visceral pain [10]. However, Kim et al. [11] have classified somatic pain as clearly localized pain limited to the peritoneal wall of the surgical site, and visceral pain as dull, nonlocalized, general, squeezing pain. They studied pain patterns in patients lying still in the supine position, and they found that after cholecystectomy by laparoscopy, somatic pain was greater than visceral pain. Also, when 0.25% bupivacaine 20 ml was locally infiltrated into the peritoneal wall before surgery, it delayed the first use of analgesics, its dosage, and the severity of pain [4].
On the other hand, Joris et al. [12] stated that visceral pain was greater than somatic pain in patients with cholecystectomy by laparoscopy. Also Pasqualucci et al. [5] stated that in cholecystectomy by laparoscopy, the administration of 0.5% bupivacaine 20 ml in the peritoneal space for visceral pain prevention against stimulation of the diaphragm by carbon dioxide and surgical manipulation was effective in pain alleviation for the first day after surgery.
In the present study our analysis did not differentiate between somatic and visceral pain. But there was not even one patient who complained of shoulder pain, which is a sign of diaphragmatic stimulation from the infusion of carbon dioxide. There was one patient who complained of scrotal pain. The areas of pain that patients severely complained of were sharp pain at the navel area where the trocar had been inserted and dull pain at the hernia site where surgical manipulation had been performed. Thus the pain patterns were diverse.
Also in inguinal hernia repair, laparoscopy is known to have lower pain than open surgery [2]. But in the pilot study there were many patients who needed opioid analgesics because of severe pain in the recovery room. Lau et al. [13] reported that 9.2% of 313 patients who had TEP experienced post-operative chronic pain, and they reported that severe post-operative pain is a risk factor for chronic pain. They stated that to deal with such acute and chronic pain, the ilioinguinal-iliohypogastric nerve block in open inguinal herniorrhaphy is blindly performed, which reduces post-operative pain and aids in early ambulation [14]. However, complications such as intestinal perforation and subserous hematoma are reported in pediatric patients [15,16]. So recently ultrasound-guided studies have been performed to increase accuracy [17]. Aveline et al. [18] reported that ultrasound-guided TAPB controls pain on the day of surgery much better than the blind ilioinguinal-iliohypogastric nerve block in open inguinal herniorrhaphy.
Because the TEP was performed in the extraperitoneal area, we assumed that the pain patients complained about was somatic pain. We applied the preemptive analgesia for abdominal surgeries suggested by the previous studies, and we expected that preoperative ultrasound-guided TAPB would continuously and completely control post-TEP pain. But the results fell short of our expectations.
In 2001, Rafi first described the traditional TAPB method, where a blunt needle was blindly inserted vertically in the 'triangle of petit' (marked by the iliac crest, external abdominal oblique muscle, and latissimus dorsi muscle), and after feeling a 'pop' when the fascia was punctured, local anesthetics were infused. McDonnell et al. [19-21] stated that when TAPB was performed with this method, they saw sensory loss from T7 to L1, and they reported that bilateral TAPB performed in caesarean sections and large bowel resections reduced post-operative pain and decreased the dosage of morphine used by patient-controlled analgesia by more than 70%. Carney et al. [22] performed unilateral TAPB with the same method in an open appendectomy and were able to reduce morphine dosage by more than 50%. However, the blind method is reported to have complications such as the needle directly damaging the liver [23], and the procedure faces drawbacks with obese patients because it is difficult to assess the depth of the needle with them.
Recently, the use of ultrasound-guides has increased. Hebbard et al. [8] introduced the mid-axillary approach where the direct location of the needle can be seen and local anesthetics is accurately injected into the TAP. With the same method, TAPBs were used for various abdominal surgeries such as laparoscopic cholecystectomies, caesarean sections, and open appendectomies; reducing the dosage of opioid analgesics and having effective results for pain control [6,24,25]. However, Tran et al. [26] performed a cadaver study using the Hebbard method and saw that only T10-L1 was stained when they used a dye 20 ml. So they stated that rather than the traditional method using the 'triangle of petit,' the ultrasound-guided mid-axillary approach could be used restrictively for lower abdominal surgery. Therefore in the present study for the lower abdominal surgery, an ultrasound-guided TAPB was performed, and the mid-axillary approach was used, which did not require a change in the patient's position after anesthesia induction. As a result we were able to reduce post-operative pain in the recovery room and the dosage of analgesics. However, we could not continuously reduce pain until post-surgery 24 hours as was accomplished in the study by Ra et al. [7].
In the study by Carney et al. [27], when TAPB was performed they used contrast media to observe the changes in an MRI over time. With the mid-axillary approach, the contrast media spread in most TAPs, and 4 hours after injection there was hardly any contrast media left. However, in the lateral position with the posterior approach between the quadratus lumborum and lateral abdominal muscles, the contrast media pooled around the quadratus lumborum and spread to the paravertebral region, and even after 4 hours the contrast media had decreased but still remained. Also among the ultrasound-guided approaches, the posterior approach had the most similar pattern to traditional TAPB in contrast media deposits.
The present study results demonstrated that we did not have a continuous and complete pain control compared to the control group. The reason is considered to be due to the fact that the mid-axillary approach, as in the afore-mentioned studies, compared to traditional approaches had a short action time and only a very small amount diffused to the paraspinal region, so its analgesic effect is considered to have been small. Another reason could be that pain reduction on the day of surgery is faster than other laparoscopic procedures [7].
In the present study, a total of ropivacaine 112.5 mg was used. When Griffiths et al. [28] performed ultrasound-guided bilateral TAPB in women who had obstetric surgeries, they administered 3 mg/kg ropivacaine diluted to 40 ml. They stated that the 30 min peak blood concentration reached a mean 2.54 ± 0.37 µg/ml. The highest concentration level in a patient was 4.00 µg/ml. Also the highest blood concentration up to 90 min was at least a mean 2.2 µg/ml. In a similar study, Latzke et al. [29] reported that after ropivacaine 150 mg was administered to healthy men, the highest blood concentration was a mean 1.88 ± 0.37 µg/ml. Combining the findings of the two studies, it seems that after TAPB, the blood concentration of ropivacaine is not affected by age, gender, or body fat, and the blood concentration ropivacaine that causes neurological symptoms in healthy men is about 2.2 µg/ml [30]. When TAPB is performed, one must always be mindful of the possibility of side effects of ropivacaine. In short surgeries the patient's condition must be carefully observed even after surgery.
Ra et al. [7] stated that ultrasound-guided TEP in cholecystectomy by laparoscopy reduced post-operative pain and dosage of analgesics compared to the control group, but there was no significant difference between the groups that used 0.25% and 0.5% bupivacaine 30 ml. We therefore used 0.375% ropivacaine 30 ml in the present study. The operative time was short, but there were no patients showing neurologic symptoms.
Low visceral pain and high somatic pain are expected with TEP. The significance of the study is that it is the first to look into the effects of the pre-operative ultrasound-guided TAPB by the mid-axillary approach on pain control in TEP.
The limitation of this study is that we performed TAPB after general anesthesia so we could not evaluate the range of the nerve block. The study by Latzke et al. [29] stated that if the administered local anesthetics do not accurately diffuse, that the concentration of the local anesthetics at the target site can be lower than the blood concentration, and the nerve block may have no effect. However, the ultrasound-guided TAPB was performed by an anesthesiologist that had experience performing more than 200 ultrasound guided nerve blocks, and we tried to administer local anesthetics to deeper fascia in TAP.
In conclusion, ultrasound-guided bilateral TAPB significantly reduced post-TEP pain in the recovery room and reduced analgesic dosage. Also it significantly reduced pain on coughing until 8 hours after surgery.