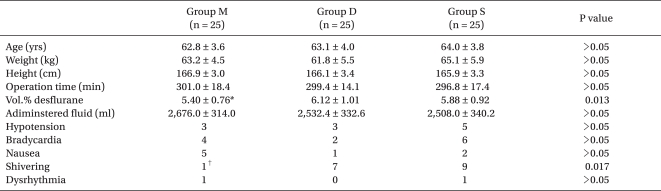
The effects of magnesium sulfate infiltration on perioperative opioid consumption and opioid-induced hyperalgesia in patients undergoing robot-assisted laparoscopic prostatectomy with remifentanil-based anesthesia
Article information
Abstract
Background
Opioids not only exert an antinociceptive effect, but also modulate central N-methyl-D-aspartate (NMDA) receptors, resulting in hyperalgesia and acute opioid tolerance. This study was aimed to investigate the effect of the NMDA receptor antagonist, magnesium in preventing remifentanil-induced hyperalgesia.
Methods
For this study, 75 patients scheduled for robot-assisted laparoscopic prostatectomy were randomly allocated into three groups of patients whose incision sites were infiltrated: Group M, with 25% magnesium sulfate 80 mg/kg; Group S, with the same volume of saline under remifentanil-based anesthesia, and Group D, with the same volume of saline under desflurane based anesthesia. All three groups were infiltrated into incision sites after pneumoperitoneum. Intraoperative evaluation included mean remifentanil dose, and postoperative evaluation included pain severity at time intervals of 30 min, 6, 12, 24 and 36 hours, time to first postoperative analgesic requirement, and analgesic dosage required during 24 hours.
Results
Mean remifentanil doses during the intraoperative periods in group M were significantly lower than those in group S (P < 0.001). The time to first postoperative analgesic requirement in postoperative period in groups M and D was significantly longer than that in group S (P < 0.001). Visual analog scale scores for pain in groups M and D were significantly lower than those in group S for 12 hours after operation.
Conclusions
A relatively high dose and continuous infusion of remifentanil were associated with opioid induced hyperalgesia. Wound infiltration with magnesium sulfate decreased opioid consumption and reduces opioid induced hyperalgesia.
Introduction
The many advantages of robot-assisted laparoscopic prostatectomy (RALP) include: better visualization, more controlled finer movements of robotic arms allowing better dissection, less blood loss and improved continence and potency rates. RALP is a rapidly growing minimally invasive surgical approach. It is becoming a standard alternative to both open and laparoscopic surgical treatment for localized prostate cancer, especially in the United States [1]. However, postoperative pain may still be moderate to severe for some patients, and up to 80% of patients require opioid analgesia after laparoscopy [2]. Because the pain comprises several components, multimodal analgesic techniques are needed for effective postoperative analgesia [3].
Remifentanil is a short-acting opioid with predictable and rapid recovery that is relatively independent of the dose and the duration. Supplemental opioids are often given prophylactically to patients who are likely to experience postoperative pain [4]. This observation suggests that remifentanil may be associated with opioid induced hyperalgesia. Magnesium is a physiological blocker of the NMDA receptor. Post-synaptic depolarization following nociceptive inputs decreases this ability to block calcium inflow through the receptor. Thus, by blocking the NMDA receptor throughout the perioperative period, magnesium might play a key role in postoperative pain, sensitization process and hyperalgesia [5]. Indeed, magnesium has been used as an effective adjuvant in postoperative pain management [6,7].
We hypothesized that intraoperative remifentanil administration results in acute opioid induced hyperalgesia (OIH) that is manifested by shortening of the time of first postoperative analgesic requirement, increased postoperative pain and opioid requirement. We therefore tested whether magnesium infiltration of incision sites in patients undergoing robot-assisted laparoscopic prostatectomy under remifentanil based anesthesia reducing intraoperative opioid consumption and opioid induced hyperalgesia.
Materials and Methods
After obtaining approval from the Institutional Review Board and written informed consent, 75 ASA I-II patients undergoing RALP were enrolled in this study. Patients were excluded if they had major hepatic, renal and cardiovascular disease, asthma, chronic obstructive pulmonary disease, as well as known allergy to magnesium sulfate or other drugs, and treatment with calcium channel blocker, opioids and anticoagulant. Patients scheduled for the operation under remifentanil-based anesthesia were randomly allocated into three groups that were infiltrated into their incision sites; group M with 25% magnesium sulfate 80 mg/kg, group S with the same volume of saline under remifentanil-based anesthesia and group D with same volume of saline under desflurane-based anesthesia after pneumoperitoneum.
All patients were premedicated with midazolam 2-3 mg before arrival in the operating room. The patients were placed routine monitors including pulse oximeter, automated cuffed blood pressure (BP), electrocardiogram (EKG), and end-tidal CO2 (ETCO2). Further, arterial and urinary catheter were placed accordingly as part of their routine management, and patients received lactated Ringer's solution for crystalloid. To determine the pre and postoperative serum magnesium, albumin, and hematocrit levels, 4 ml of arterial blood samples were withdrawn from patients after induction of general anesthesia and at the end of surgery and analyzed immediately.
Induction of anesthesia was commenced with a slow (30-60 s) i.v. bolus dose of remifentanil 1 µg/kg followed by propofol 1 mg/kg for group M and group S or, with propofol (2 mg/kg) in group D: tracheal intubation was facilitated with rocuronium (0.9 mg/kg) in all groups. After intubation of trachea, the patients are ventilated to normocapnia with 50% oxygen without nitrous oxide and, end-tidal desflurane concentration was maintained at 1 minimum alveolar concentration (MAC) according to age in groups M and S. The anesthesia level was monitored using the BIS method in all groups, as well as heart rate and blood pressure. The BIS electrodes were placed on the forehead and connected to BIS monitoring system (BIS XP, A-2000, Aspect Medical Systems, USA). BIS was maintained between the range of 40 to 60 during operation for three groups. Our criteria for possibly insufficient anesthesia were a heart rate that exceeded preinduction values by 15% and/or a systolic blood pressure that exceed baseline values by 20% for at least 5 min.
Remifentanil was started at a rate of 0.3 µg/kg/min in group S and 0.1 µg/kg/min in group M and subsequently stepwise by 0.05 µg/kg/min increments if inadequate anesthesia was suspected. Group D patients received the same volume of saline in place of remifentanil, and the concentration of desflurane was titrated to prompted stepwise 1% reductions in desflurane concentration. The patients received 10 mg i.v. bolus doses of ephedrine, in case of persistent hypotension. If the heart rate decreased to less than 50 beats/min, 0.5 mg i.v. atropine bolus was administered.
The patients were placed in Trendelenburg position after anesthesia induction. All potential pressure points were protected with foam padding. The robot was parked between the abducted, slightly flexed legs supported by stirrups. Pneumoperitoneum was maintained throughout the procedure (15 mmHg). The camera port (12 mm), 2 robotic arm ports (8 mm) and 2 additional working ports (12 mm, for retraction, suction, and introduction and cutting of sutures) were carefully planned according to the geometry of the anatomic landmarks.
At the end of surgery, neuromuscular blockade was reversed with pyridostigmine 0.2 mg/kg, glycopyrrolate 0.008 mg/kg when the TOF ratio had returned to 25%. When BIS values reached 80 and spontaneous breathing was achieved, extubation was performed. Remifentanil infusion was discontinued when the last surgical stitch was placed. Every patients were administered analgesics by patient-controlled analgesia (PCA) pump (Accufusor® WooYoung medical, Korea) containing morphine (40 mg), ketorolac (180 mg), and ramosetron (0.6 mg) in normal saline in a total volume of 100 ml. This device was set to deliver a basal infusion 2 ml/hr, a bolus dose of 0.5 ml with a 15 min lockout time. Pain scores at movement were documented using the 100 mm linear visual analog scale (VAS) which is a straight line with the left end of the line representing no pain and the right end of the line representing the worst pain. Patients are asked to mark on the line where they think their pain is. Intraoperative evaluation included time weighted mean remifentanil dose, the age-adjusted desflurane concentration, and postoperative evaluation included degree of pain severity at 30 min, and 6, 12, 24 and 36 hours, the time to first postoperative analgesic (pethidine or ketorolac) requirement, and analgesic dose required during the first 24 hours. Side effects including hypotension, bradycardia, nausea, and shivering were also recorded. All patients were monitored in the postoperative recovery room for the first 24 hours.
Postoperative monitoring included noninvasive blood pressure, heart rate, and pulse oximetry. Nausea was treated with i.v. metoclopromide (10 mg), and postoperative shivering was treated with a forced air warming blanket. A trained anesthesiologist who was not involved in this study assessed pain, and analgesic consumption. An i.v. dose of pethidine 25 mg if patients reported VAS ≥ 50 or i.v. dose of ketorolac 30 mg if VAS < 50 were administered during recovery for 24 hours.
A preliminary investigation showed that hypothesized means of time to first postoperative analgesic requirement in postoperative period in group M, group D and group S were 45.3 min, 43.2 min and 36.1 min respectively, and standard deviation of subjects was 10.3. Sample size of 24 patients per group is needed to demonstrate a significant difference, with power of 80% and an α-coefficient of 0.05. The results are presented as mean ± SD or the number of patients. Comparisons of age, body weight, operation time, vol% of desflurane, the time to first postoperative analgesic requirement, fluid administered, VAS scores for pain and analgesics administered among groups were conducted by using One-way ANOVA. Time-weighted mean remifentanil dose between Groups M and S was conducted by using an unpaired t-test. Chi-Square test was used to analyze categorical data such as hypotension, bradycardia, nausea, shivering and dysrhythmia. P < 0.05 was considered the minimum level of statistical significance.
Results
The three groups were comparable with respect to age, height, weight, fluid administered, hypotension, bradycardia, nausea, and dysrhythmia. Desflurane concentration administered was significantly lower in group M than in group D. Shivering in group M was significantly lower than that in other groups (Table 1).
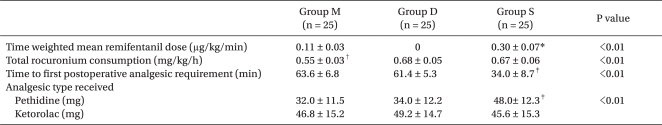
Time weighted mean remifentanil dose in group S was significantly greater than that of group M. Total rocuronium consumption of group M was significantly lower than that in group D and group S. Time to first operative analgesic requirement of group S was significantly shortest than those of group M and group D, but there was no significant difference between groups M and D. The pethidine dose administered for 24 hours in group S was significantly greater than those in groups M and D, but there was no significant difference between groups M and D (Table 2). Postoperative cumulative injected volume of the PCA pump in group S was significantly greater than those in groups M and D, but there was no significant difference between groups M and D (Table 3).
There were no significant differences in preoperative and postoperative total magnesium and albumin level in all groups (Fig. 1 and 2). Postoperative hematocrit was significantly decreased to preoperative hematocrit in all groups (Fig. 3).
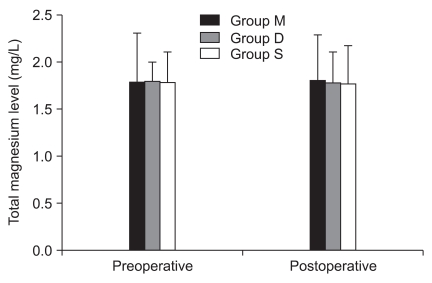
Preoperative and postoperative serum magnesium. Values are mean ± SD. Group M: 25% magnesium sulfate (80 mg/kg) with remifentanil. Group S: same volume of saline with remifentanil. Group D: same volume of saline with desflurane. There were no significant differences among groups.

Preoperative and postoperative serum albumin. Values are mean ± SD. Group M: 25% magnesium sulfate (80 mg/kg) with remifentanil. Group S: same volume of saline with remifentanil. Group D: same volume of saline with desflurane. There were no significant differences among groups.
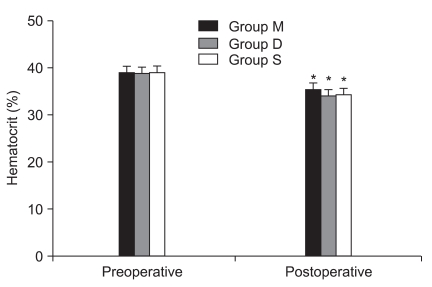
Preoperative and postoperative hematocrit. Values are mean ± SD. Group M: 25% magnesium sulfate (80 mg/kg) with remifentanil. Group S: same volume of saline with remifentanil. Group D: same volume of saline with desflurane. *P < 0.05 versus preoperative hematocrit in each group.
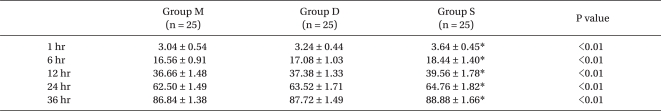
VAS scores for pain were significantly higher in group S than in group M for 24 hours and in group D for 12 hours after operation, but there was no significant difference between groups M and D (Fig. 4).
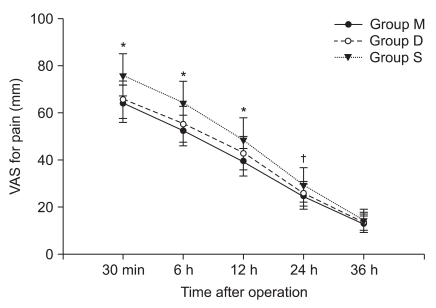
Sequential changes of VAS pain scores during movement in three groups after operation. VAS: visual analog scale. Values are mean ± SD. Group M: 25% magnesium sulfate (80 mg/kg) with remifentanil. Group S: same volume of saline withremifentanil. Group D: same volume of saline with desflurane. *P < 0.05 versus group M and group D. †P < 0.05 versus group M.
Discussion
The results of this study demonstrated that there was no significant difference in desflurane concentration between groups S and D, and group S who received a continuous intraoperative remifentanil compared with group D who received saline resulted in acute opioid induced hyperalgesia (OIH). OIH in this study was manifested by the shortening of time of first postoperative analgesic requirement, increased postoperative pain, and opioid requirement. Group M reduced OIH which was shown in group S.
OIH is most broadly defined as a state of nociceptive sensitization caused by exposure to opioid. The state is characterized by a paradoxical response where one actually become more sensitive to certain painful stimuli. In clinical setting, OIH may represent one of many reasons for declining levels of analgesia while receiving opioids or worsening pain syndrome. This phenomenon is thought to result from neuroplastic changes in the central nervous system or peripheral nervous system leading to sensitization of pronociceptive pathway [8]. Conflicting results have been published concerning the effect of intraoperative remifentanil dose on postoperative pain sensitivity. Two prospective controlled clinical studies have reported increased postoperative pain despite increased postoperative opioid [9,10]. A study by Joly et al. [11] directly measured the development of secondary wound hyperalgesia after acute intraoperative opioid exposure. The authors found that high-dose intraoperative exposure to the potent, ultra short-acting µ-opioid agonist remifentanil increased periincisional wound allodynia and hyperalgesia measured by von Frey hairs compared with low-dose intraoperative remifentanil in patients undergoing major abdominal surgery. These findings are contrasted by other studies that did not show an effect of intraoperative opioid dose on postoperative pain sensitivity. Cortínez et al. [12] did not find increased pain or postoperative opioid consumption after high-dose intraoperative remifentanil exposure in patients undergoing elective gynecologic surgery. Additionally, Lee et al. [13] also did not see a significant difference in postoperative pain or opioid consumption in patients who received intraoperative remifentanil compared with 70% nitrous oxide after colorectal surgery. Opioids not only exert an antinociceptive effect but also modulate central NMDA receptors, resulting in hyperalgesia and acute opioid tolerance. NMDA receptor antagonists such as ketamine or magnesium, have been demonstrated to increase the analgesic effects of opioids, probably by limiting these NMDA-mediated facilitating processes [14].
Magnesium can then lead to a decrease in postoperative pain and analgesic consumption, and has been used as an effective adjuvant in postoperative pain management [5-7].
It has been reported that volatile anesthetics including desflurane block the NMDA receptor [15,16]. However, Hollmann et al. [15] reported that desflurane inhibits control response of NMDA receptor to glutamate only by 20% at 0.5 MAC and 40% at 1 MAC. This results support that a small difference in desflurane concentration does not explain the exaggerated hyperalgesia in group S which received a large dose of remifentanil.
The development of hypomagnesemia has been reported in 60-70% of postoperative patients [17,18]. There is a evidence that the response of the NMDA receptor is greatly enhanced by reducing the extracellular magnesium concentration below physiologic levels [19]. However, this study showed that there were no significant differences in preoperative and postoperative total magnesium level among groups, although group S and group D showed reduction of magnesium in postoperative period. This result suggests that magnesium sulfate does not act through its systemic action [20]. The mechanism of peripherally administered magnesium sulfate remain unclear. Some studies identified perial NMDA receptor in the skin and muscles [21,22]. Tauzin-Fin et al. [23] recently reported that co-administration of magnesium sulfate with ropivacaine for postoperative infiltration analgesia after radical retropubic prostatectomy procedures provided a significant reduction in opioid requirement. Magnesium provides anesthetic, analgesic, anti-shivering and muscle-relaxant effects.
This study showed that time to first operative analgesic requirement, pethidine dose administered, VAS scores for pain, postoperative cumulative injected volume of PCA pump, shivering and rocuronium consumption in group M were significantly better compared with those in group S, but pain-related clinical variances were no significant when compared with group D. Based on these findings, we suggest that magnesium itself do not produces antinociceptive effects but prevents opioid induced hyperalgesia. In clinical practice, magnesium's beneficial effect on shivering is of minor importance compared with the efficacy that was reported with meperidine or clonidine [24,25].
In conclusion, we confirmed our hypothesis that intraoperative remifentanil administration results in acute OIH that is manifested by shortening of the time of first postoperative analgesic requirement, increased postoperative pain and opioid requirement in group S under remifentanil based anesthesia compared with group D under desflurane based anesthesia. There were no advantages in group S (combined desflurane and remifentanil) over group D (desflurane only) as far as pain, although remifentanil-based anesthesia has been known to have an advantage in a predictable and rapid recovery. Magnesium infiltration of incision sites in patients undergoing robotic-assisted laparoscopic prostatectomy under remifentanil-based anesthesia reduces intraoperative opioid consumption and opioid induced hyperalgesia. Further studies are required to obtain more informations about safety and to determine whether doses of magnesium sulfate can provide postoperative analgesic benefits.
Acknowledgements
This study was supported by Wonkwang University 2011.