 |
 |
| Korean J Anesthesiol > Volume 76(6); 2023 > Article |
|
Abstract
Background
Cesarean section is associated with moderate to severe pain and nonsteroidal anti-inflammatory drugs (NSAIDs) are commonly employed. The optimal NSAID, however, has not been elucidated. In this network meta-analysis and systematic review, we compared the influence of control and individual NSAIDs on the indices of analgesia, side effects, and quality of recovery.
Methods
CDSR, CINAHL, CRCT, Embase, LILACS, PubMed, and Web of Science were searched for randomized controlled trials comparing a specific NSAID to either control or another NSAID in elective or emergency cesarean section under general or neuraxial anesthesia. Network plots and league tables were constructed, and the quality of evidence was evaluated with Grading of Recommendations Assessment, Development and Evaluation (GRADE) analysis.
Results
We included 47 trials. Cumulative intravenous morphine equivalent consumption at 24 h, the primary outcome, was examined in 1,228 patients and 18 trials, and control was found to be inferior to diclofenac, indomethacin, ketorolac, and tenoxicam (very low quality evidence owing to serious limitations, imprecision, and publication bias). Indomethacin was superior to celecoxib for pain score at rest at 8–12 h and celecoxib + parecoxib, diclofenac, and ketorolac for pain score on movement at 48 h. In regard to the need for and time to rescue analgesia COX-2 inhibitors such as celecoxib were inferior to other NSAIDs.
Cesarean section is one of the most common operations performed worldwide. It is, however, associated with moderate to severe pain in almost four fifths of women [1] and, when compared to many other surgical procedures, it has been reported to be the ninth most painful operation on the first postoperative day [2]. Pain during and following cesarean section has been demonstrated to be of greatest concern to women [3], and inadequate pain relief has been related to negative effects on breastfeeding and infant care [1], maternal dissatisfaction [4], postpartum depression [5], and chronic pain [5,6].
Nonsteroidal anti-inflammatory drugs (NSAIDs) are commonly used as part of a multimodal strategy in the perioperative period, and provide analgesia by the inhibition of cyclooxygenase enzymes that are involved in the formation of hyperalgesic prostaglandins [7]. In a meta-analysis that compared control to NSAIDs, NSAIDs decreased the pain score at rest at 12 h and 24 h and on movement at 24 h, lowered opioid consumption, and reduced the risk of sedation, the latter a recognized side effect of opioids [8]. Given this, the procedure specific postoperative pain management (PROSPECT) recommendations for elective cesarean section include the intraoperative use of intravenous NSAIDs and postoperative use of oral or intravenous NSAIDs [9]. It is still not clear, however, which NSAID is most effective in the setting of cesarean section. Different NSAIDs may produce varying pain relief efficacy and have differing side effect profiles, and hence a comparative analysis of NSAIDs is important. Several randomized trials investigating NSAIDs have been published recently [10,11], and a contemporary review would update the available evidence for the use of NSAIDs in cesarean section.
Our aim in this network meta-analysis and systematic review was to compare the influence of control and individual NSAIDs such as diclofenac and ibuprofen on the indices of analgesia, side effects, and quality of recovery. We hypothesized that we would establish the overall efficacy of NSAIDs in cesarean section, and potentially uncover differences among the NSAIDs studied.
We prospectively registered the protocol for the systematic review and network meta-analysis with PROSPERO (CRD42021264209), and our findings have been presented in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [12]. The following databases, CDSR, CINAHL, CRCT, Embase, LILACS, PubMed, and Web of Science, were searched from inception to May 27, 2021, for free text keywords and subject headings associated with different permutations of terms related to cesarean section, obstetric analgesia, NSAIDs in general, and specific NSAID drug names (Supplementary Material 1).
Once duplicate citations were discarded, two authors (IM and ND) independently screened the titles and abstracts of the remaining citations against the inclusion and exclusion criteria in Rayyan (Qatar Computing Research Institute, 2016, Doha, Qatar) [13]. Inclusion criteria were defined as randomized controlled trials that compared a specific NSAID to either control or another NSAID in the context of elective or emergency cesarean section under general or neuraxial anesthesia. The timing of NSAID administration could be preoperative, intraoperative, and/or postoperative, and trials that investigated more than one NSAID, either combined or in more than one arm, were included. Exclusion criteria included trials in which regional anesthesia or wound catheters were utilized postoperatively. Trials that included intraoperative local anesthetic infiltration and single-shot transversus abdominis plane block, for example, were included, but those that used postoperative infusions of local anesthetic through catheters into the epidural space, transverse abdominis plane, or wound were excluded. No limits were placed on the language of publication. Cases of disagreement were resolved by a third author (BC). If a trial was thought to be eligible for inclusion, then we carried out a full text review to confirm this. In order to seek further trials not identified by our search strategy, one author (AC) searched the reference lists of included trials and previously published systematic reviews.
Data extraction was conducted and checked by five authors (IM, AC, PS, JO, and ND). The following characteristics of trials were extracted: number of patients in each group; nature of cesarean section; mode of anesthesia; intraoperative regional anesthesia and systemic analgesia; dose, route, and timing of NSAID administration; regular postoperative analgesia; and management of postoperative breakthrough pain. The primary outcome was the cumulative intravenous morphine equivalent consumption at 24 h, and the MCID was prespecified at 10 mg. It is the opinion of the authors that this outcome is particularly important as it provides a measure of pain and need for rescue analgesia on the first postoperative day, and increased opioid consumption has been associated with side effects such as nausea and vomiting, urinary retention, constipation, and sleep disturbance that can lead to distress and interfere with postoperative recovery [14]. In a systematic review, the clinician perceived the MCID estimate for this primary outcome in the setting of total hip and knee arthroplasty was 10 mg and, in the absence of evidence-based and patient-rated MCIDs, we concurred with this [15]. Secondary outcomes included: pain score at rest and on movement at 8–12 h, 24 h, and 48 h; need for rescue analgesia and time to first analgesic request; cumulative intravenous morphine consumption at 8–12 h, 48 h, and in-hospital; incidence of postoperative nausea and/or vomiting, pruritus, and sedation at 24 h, 48 h, and in-hospital; quality of recovery-15 (QoR-15) [16] at 24 h and 48 h; and hospital length of stay. No other secondary outcomes were considered. We extracted dichotomous data as numbers and continuous data as means and standard deviations. If data were presented as medians, these were assumed to be equal to the means, and the standard deviations were calculated by dividing the interquartile range by 1.35 or the range by 4 as per guidance from the Cochrane Collaboration [17]. In cases where data were presented only in graphical format, PlotDigitizerTM (Version 2.1, Free Software Foundation, USA) was utilized in order to facilitate numerical extraction. Opioid conversion was performed with reference to the British National Formulary [18] and Faculty of Pain Medicine [19]. Where the data were not published or unclear, the authors were emailed up to three times for clarification.
Subsequent to data extraction, the data were transferred from Microsoft Excel® (Microsoft, USA) into Stata (Version 16.1, StataCorp LLC, USA) by one author (ND) and then checked by a second author (IM). We conducted this network meta-analysis with a frequentist method on any outcome of interest if three or more competing interventions could be connected into a network through direct comparisons between the trials [20,21]. Network plots were produced for all outcomes subjected to network meta-analysis with a common heterogeneity parameter and multivariate methods. In these network plots, the nodes depicted the interventions and the connecting lines represented the direct comparisons between the interventions. If interventions were not directly compared within trials, indirect comparisons via a common comparator were mathematically derived using results from the various direct intervention effects. Consistency was locally and globally assessed between direct and indirect estimates by the Separating Indirect from Direct Evidence technique and with the design-by-treatment interaction test, respectively. The results of comparisons between the different interventions were presented in network league tables as mean differences and 95% CIs for continuous outcomes and odds ratios and 95% CIs for dichotomous outcomes. If serious imprecision was not present for a particular outcome, competing interventions were ranked in order. We performed pairwise meta-analysis in Review Manager® (Version 5.3, The Nordic Cochrane Centre, Denmark) for those outcomes that were not analyzable by network meta-analysis but were reported by two or more randomized controlled trials. Heterogeneity was calculated with predetermined thresholds for low (25%–49%), moderate (50%–74%), and high (≥ 75%) levels [22], and the fixed and random effects model used for low and moderate or high heterogeneity, respectively. Tests were two-tailed and statistical significance was represented at the 5% level. The results were presented as mean differences and 95% CIs for continuous outcomes and risk ratios and 95% CIs for dichotomous outcomes.
The quality of evidence for every outcome was evaluated by two authors (IM and ND) using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system [23] and with the CINeMA software® (Institute of Social and Preventative Medicine, University of Bern, Switzerland). Fundamental components of quality include: risk of bias, indirectness, imprecision, inconsistency, and publication bias. Risk of bias was determined by two authors (JO and DO) using the Cochrane Risk of Bias 2 (RoB 2) tool [24] to examine the following: randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result. Cases of disagreements were resolved by a third author (ND). Publication bias was examined with a comparison-adjusted funnel plot and the Egger’s linear regression test.
In all, we included 47 trials in this review [10,11,25–69] and details of the screening process are illustrated in Fig. 1. The following interventions were compared: control vs. celecoxib in two trials [35,46]; control vs. celecoxib + parecoxib in one trial [57]; control vs. diclofenac in 24 trials [11,25–34,36–43,65–69]; control vs. diclofenac vs. indomethacin in one trial [44]; control vs. diclofenac vs. ketoprofen in one trial [45]; control vs. ibuprofen vs. ketorolac in one trial [47]; control vs. indomethacin in one trial [48]; control vs. ketorolac in six trials [10,49–53]; control vs. naproxen in one trial [54]; control vs. parecoxib in one trial [55]; control vs. tenoxicam in six trials [56,58–62]; diclofenac vs. ketoprofen in one trial [63]; and ketorolac vs. parecoxib in one trial [64]. The findings of the risk of bias assessment are presented in Fig. 2. Overall, only four trials were deemed to be at low risk of bias [10,54,55,68], and 30 and 13 of the remaining trials were evaluated to have some concerns [25,27–35,37,38,43–45,47,49,52,53,57–67] or be at high risk of bias [11,26,36,39–42,46,48,50,51,56,69], respectively. Many of the concerns were related to the randomization process, measurement of the outcome, and the selection of the reported result. Of the 21 authors we emailed to clarify on methodology or results, nine responded with the requested information [38,42,43,50,53,55,61,62,65].
Characteristics of the trials are presented in Table 1. In regard to the nature of the cesarean section, it was elective, elective or emergent, and not specified in 30 [10,26,29,31–35,37,38,45,48–51,53–58,60–62,64–69], six [11,30,40–43], and 11 [25,27,28,36,39,44,46,47,52,59,63] trials, respectively. The mode of anesthesia was spinal, combined spinal-epidural (CSE), epidural, or general anesthesia in 27 [10,11,29,32–35,37–39,41–44,46,48,50,54–56,60–64,68,69], two [51,57], three [49,66,67], and 11 [25–28,30,31,47,53,58,59,65] trials, respectively. Of the remaining trials, one performed spinal or epidural anesthesia [45], two used neuraxial or general anesthesia [40,52], and one did not specify the type of anesthesia [36]. Single-shot transversus abdominis plane block was utilized in one trial [11]. In addition to NSAIDs, women received propacetamol or paracetamol in four trials [32,33,37,57]. The route of administration of NSAIDs was as follows: oral in two trials [35,46]; intramuscular in 13 trials [25,27,28,31,34,36,38,40,43,49,65–67]; intravenous in 16 trials [10,11,39,45,50–53,55,56,58–62,64]; rectal in 11 trials [26,29,30,32,33,41,42,44,48,68,69]; oral or intramuscular in one trial [47]; intravenous and oral in one trial [57]; intramuscular or intravenous in one trial [63]; and rectal and oral in two trials [37,54]. In 21 trials, just one dose of NSAIDs was administered [10,11,26,35,36,39,46,49,55,56,58–63,65–69] and in further 21 trials, more than one dose or an infusion of NSAIDs was given [25,29–33,37,38,40,42–45,48,50–54,57,64]. Some trials provided NSAIDs only when the pain was reported to be at least moderate in intensity [34,41], or the pain score was greater than or equal to seven on a scale of zero to 10 [27,28] or higher than or equal to 60 on a scale of zero to 100 [47].
Our primary outcome, the cumulative intravenous morphine equivalent consumption at 24 h, was evaluated in 1,228 patients and 18 trials [27–29,32–34,37,39,44–46,50,52,55,58,61,62,64]. In the network plot, nine direct and 12 indirect comparisons were established between seven interventions (Fig. 3). With an MCID of 10 mg, control was clinically and statistically inferior to diclofenac, indomethacin, ketorolac, and tenoxicam (Table 2). No other statistical differences were demonstrated between the various NSAIDs. Evidence for local or global inconsistency was not found and the standard deviation of between-trials heterogeneity was 11.08. Inspection of the comparison-adjusted funnel plot (Supplementary Fig. 1) and the results of Egger’s test (P = 0.011) revealed the presence of publication bias. The quality of evidence was graded as very low (Supplementary Material 2), and the network ranking of interventions was not performed in view of the serious imprecision (Supplementary Material 3).
Details of the results of the secondary outcomes are presented in Table 3 and Supplementary Material 3, and information related to their network plots, inconsistency plots, contribution plots, predictive interval plots, and comparison-adjusted funnel plots is provided in Supplementary Material 4. Differences between NSAIDs were shown for some of these outcomes. For the pain score at rest at 8–12 h, indomethacin was clinically and statistically superior to celecoxib, and for the pain score on movement at 48 h, indomethacin was clinically and statistically superior to celecoxib + parecoxib, diclofenac, and ketorolac. In regard to the need for rescue analgesia, ketoprofen was clinically and statistically superior to celecoxib + parecoxib, and with respect to the time for rescue analgesia, diclofenac, ibuprofen, indomethacin, and ketorolac were clinically and statistically superior to celecoxib. In terms of side effects, ketoprofen was clinically and statistically superior to celecoxib + parecoxib for the rate of in-hospital pruritus, and diclofenac was clinically and statistically superior to control for the rate of sedation at 24 h and in-hospital. The hospital length of stay was statistically but not clinically different between diclofenac and control.
Our systematic review and network meta-analysis demonstrated that, compared to control, the administration of diclofenac, indomethacin, ketorolac, or tenoxicam led to a clinically significant decrease in the primary outcome, namely cumulative morphine consumption at 24 h, using an MCID of 10 mg. The quality of evidence, however, was very low due to serious limitations, imprecision, and publication bias. Differences between various NSAIDs were found, with indomethacin clinically superior to celecoxib and celecoxib + parecoxib, diclofenac, and ketorolac for the pain score at rest at 8–12 h and the pain score on movement at 48 h, respectively, when an MCID of 10 on a pain scale of 0–100 was applied. Indomethacin may be preferable, although it must be recognized that the evidence for other NSAIDs continues to emerge and is currently limited by the presence of imprecision.
In contrast to diclofenac, indomethacin, ketorolac, and tenoxicam, control was not inferior to other NSAIDs such as celecoxib and parecoxib for the cumulative morphine consumption at 24 h. It is likely that this could be a reflection of the limited evidence base for these NSAIDs, resulting in imprecision and wide CIs, and the different dosing regimens employed in the included trials. In many trials that investigated diclofenac, indomethacin, and ketorolac, more than one dose of the NSAID was administered in 24 h [25,27–33,37,38,40,42–45,48,50–53]. Further, tenoxicam has a long mean elimination half-life of 67 h [70], explaining its beneficial effects on morphine consumption despite only being given once in the relevant trials [56,58–62]. Similarly, the various dosing strategies in the included trials may explain, at least in part, the superiority of ketoprofen to celecoxib + parecoxib in regard to the need for rescue analgesia and diclofenac, indomethacin, and ketorolac, but not ibuprofen, over celecoxib with respect to the time to rescue analgesia. Selective COX-2 inhibitors such as celecoxib have gained popularity as effective analgesics, particularly as they can produce fewer gastrointestinal side effects [71]. Their inferiority to other NSAIDs could be indicative of their slow absorption from the small intestine following oral administration [72], and their relatively homogenous distribution in body tissue in comparison to acetic acid derivatives with acidic functional groups such as diclofenac, ketorolac, and indomethacin. NSAIDs that are acetic acid derivatives as well as those with high protein binding can selectively accumulate and persist in areas of inflammation [72,73], and this may facilitate their increased analgesic effectiveness at sites of tissue injury subsequent to cesarean section. The superiority of indomethacin to other NSAIDs might be representative of its potential to act as a positive allosteric modulator at the type one cannabinoid receptor, modifying the endocannabinoid system and increasing its antinociceptive properties [74].
In terms of side effects, diclofenac compared to control resulted in decreased sedation at 24 h and in-hospital. This probably underlines its capacity to influence the pain score at 8–12 h and 24 h as well as the need for and time to rescue analgesia, hence reducing the cumulative morphine consumption and these secondary undesirable effects. Interestingly, in the absence of differences in the cumulative morphine consumption, ketoprofen decreased the rate of in-hospital pruritus compared to celecoxib + parecoxib. NSAIDs do not have any recognized direct antipruritic effects [75], and it is possible that the lack of difference in the cumulative morphine consumption was once again a reflection of imprecision rather than absence of true underlying differences.
Our findings corroborate and expand upon the systematic reviews and meta-analyses conducted to date. Consistent with what we have shown, in a prior meta-analysis of 22 randomized controlled trials, NSAIDs were reported to be superior to control in the context of cesarean section for the pain score at 12 h and 24 h and the cumulative morphine consumption [8]. NSAIDs have been compared in settings outside that of cesarean section in other systematic reviews [76–78]. In a previous network meta-analysis of 26 randomized controlled trials, etoricoxib was superior to celecoxib, ketoprofen, and tenoxicam for pain relief in ankylosing spondylitis [76], and in a prior systematic review of 76 randomized controlled trials, diclofenac, etoricoxib, and rofecoxib were ranked highest for the reduction of pain in hip and knee osteoarthritis [77].
We acknowledge the limitations related to this meta-analysis. First, there were a limited number of trials comparing different NSAIDs. Second, few trials were evaluated to be at low risk of bias, and concerns were present in the remaining trials in regard to the randomization process, measurements of the outcome, and the selection of the reported result. Third, the included trials investigated patients who had emergency and/or elective cesarean section under neuraxial, with or without intrathecal opioids, or general anesthesia. Moreover, the strategy of NSAID administration was inconsistent with varied dosing, route, and duration. Such variability introduces heterogeneity, although it increases the generalizability of the findings. Fourth, the standard practice of multimodal analgesia with paracetamol was, surprisingly, only used in a minority of trials. The combination of paracetamol and NSAIDs has been recommended due to their additive effect [79,80]. Fifth, a change in the pain score of 10 on a pain scale of 0–100, including in obstetrics, has been revealed to represent a clinically important difference in the intensity of pain [81]. It is likely, however, that the MCID for any individual patient may vary depending on the severity of the pain, with a greater MCID needed for more severe pain [82]. The MCID for many indices remains undetermined in cesarean section [83], and the authors thus used their experience in this systematic review to select the different thresholds for clinical significance. Sixth, concerns with respect to imprecision for most outcomes precluded the ranking of various NSAIDs. Last, we did not examine which NSAID was best in terms of minimizing transfer to breast milk and increasing safety in breastfeeding women. Those NSAIDs with low oral bioavailability, high protein binding, short half-life, and inactive metabolites as well as reassuring data on breast milk transfer and long record of safe use are likely to be optimal in this respect [80,84]. Interestingly, ibuprofen is thought to be the ideal NSAID for women who are breastfeeding, but our results do not provide sufficient data to confirm its superior properties in the context of cesarean section.
Our network meta-analysis and systematic review demonstrated that diclofenac, indomethacin, ketorolac, and tenoxicam compared to control decreased cumulative morphine consumption at 24 h. No differences were found between different NSAIDs in the cumulative morphine consumption at 24 h, and the quality of evidence was very low. Differences in the secondary outcomes between various NSAIDs were uncovered, with indomethacin clinically superior to celecoxib and celecoxib + parecoxib, diclofenac, and ketorolac for the pain score at rest at 8–12 h and the pain score on movement at 48 h. In light of this emerging but limited evidence, our review suggests the presence of minimal differences among the NSAIDs studied. Nonselective NSAIDs may be more effective than selective NSAIDs, and some NSAIDs such as indomethacin might be preferable to other NSAIDs. Further trials with designs relevant to modern obstetric anesthesia practice are required to increase the strength and quality of the evidence base and the recommendations related to the selection of NSAIDs in the setting of cesarean section.
Acknowledgments
PS is an Arline and Pete Harman Endowed Faculty Scholar of the Stanford Maternal and Child Health Research Institute.
NOTES
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author Contributions
Iona Murdoch (Data curation; Formal analysis; Writing – original draft)
Anthony L Carver (Data curation; Writing – original draft)
Pervez Sultan (Data curation; Writing – review & editing)
James E O’Carroll (Data curation; Formal analysis; Writing – review & editing)
Lindsay Blake (Data curation)
Brendan Carvalho (Conceptualization; Writing – review & editing)
Desire N. Onwochei (Formal analysis; Writing – review & editing)
Neel Desai (Conceptualization; Data curation; Formal analysis; Writing – original draft; Writing – review & editing)
Supplementary Materials
Supplementary Material 2.
GRADE quality of evidence assessment for each outcome.
Supplementary Material 3.
Network league table for secondary outcomes.
Supplementary Fig. 1.
Comparison-adjusted funnel plot with respect to the network for cumulative morphine equivalent consumption at 24 h. Different colors correspond to particular comparisons of interventions. The red line indicates the null hypothesis that the comparison-specific pooled effect estimates do not differ from the respective trial-specific effect sizes.
Fig. 1.
PRISMA flow diagram summarizing the retrieved, included, and excluded randomized controlled trials. PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
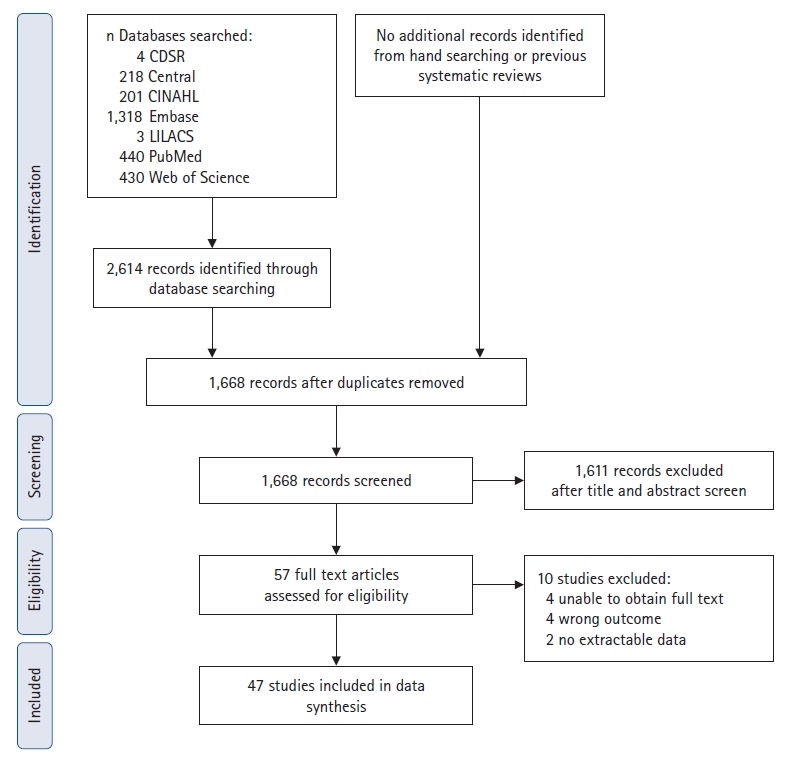
Fig. 3.
Network plot in regard to the need for cumulative intravenous morphine equivalent consumption at 24 h. Each intervention is depicted by a circle that is proportional in size to the number of patients who were randomized to that intervention. Connecting lines between the circles indicate the direct comparisons of interventions, their width proportional to the number of trials evaluating the comparison, and their color representing the average risk of bias. Green: low risk, yellow: some concerns, red: high risk.
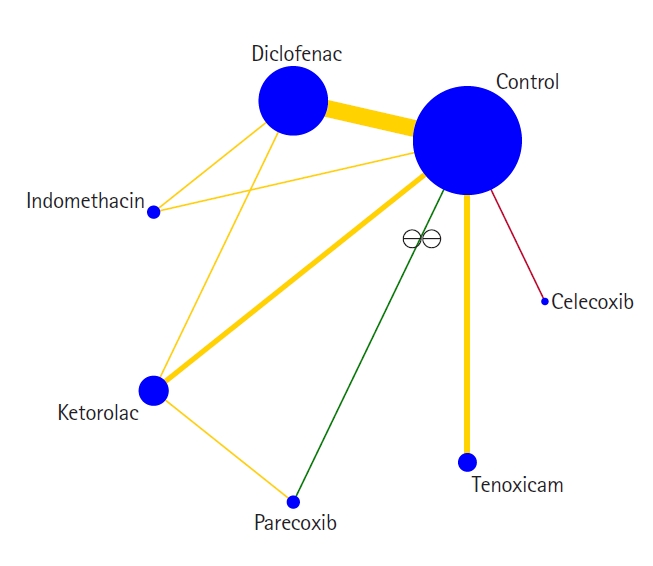
Table 1.
Characteristics of the Included Trials
| Reference | Group (n) | Journal title | Language | Country of the enrolled patients | Nature of cesarean section | Mode of anesthesia | Intraoperative regional anesthesia and systemic analgesia | Dose, route, and timing of NSAID administration | Regular postoperative analgesia | Management of postoperative breakthrough pain |
|---|---|---|---|---|---|---|---|---|---|---|
| Control vs celecoxib | ||||||||||
| Lee et al. 2004 [35] | Control (30) | Anaesthesia | English | Hong Kong | Elective; Pfannensteil approach | Spinal | Spinal: 3 ml 0.5% hyperbaric bupivacaine with morphine 300 μg | P.O. celecoxib 200 mg, once only, following delivery of neonate | Not specified | P.O. paracetamol and dextropropoxyphene |
| Celecoxib (30) | ||||||||||
| Fong et al. 2008 [46] | Control (20) | British Journal of Anaesthesia | English | Taiwan, Republic of China | Not specified | Spinal | Not specified | P.O. celecoxib 400 mg, once only, either 30 min before spinal anesthesia or following surgical wound closure | I.V. morphine PCA | Not specified |
| Celecoxib (40) | ||||||||||
| Control vs celecoxib and parecoxib | ||||||||||
| Paech et al. 2014 [57] | Control (55) | Anaesthesia and Intensive Care | English | Australia | Elective; Pfannensteil approach | CSE | CSE: 2.1–2.5 0.5% hyperbaric bupivacaine with fentanyl 15 μg | I.V. parecoxib 40 mg following delivery of neonate and P.O. celecoxib 400 mg at 12 h | P.O. paracetamol 1 g at 6, 12, and 18 h in only some groups. | P.O. tramadol |
| Celecoxib and parecoxib (56) | Systemic: I.V. paracetamol 2 g in only some groups | Patient-controlled epidural analgesia with bolus of pethidine 20 mg and lockout interval of 15 min | ||||||||
| Control vs diclofenac | ||||||||||
| Bush et al. 1992 [65] | Control (25) | Anaesthesia | English | United Kingdom | Elective; Pfannensteil approach | General anesthesia | Systemic: I.V. papaveretum 0.3 mg/kg | I.M. diclofenac 75 mg, once only, prior to discontinuation of general anesthesia | I.V. papaveretum PCA | Opioid, otherwise not specified |
| Diclofenac (23) | ||||||||||
| Sun et al. 1992 [66] | Control (58) | Anesthesia and Analgesia | English | Taiwan, Republic of China | Elective; surgical approach not specified | Epidural | Epidural: 2% lidocaine with adrenaline 5 μg/ml of unspecified volume, followed by morphine 2 mg in only some groups subsequent to delivery of placenta | I.M. diclofenac 75 mg, once only, on arrival to recovery | Not specified | I.M. pethidine |
| Diclofenac (59) | ||||||||||
| Sun et al. 1993 [67] | Control (20) | Anesthesia and Analgesia | English | Taiwan, Republic of China | Elective; surgical approach not specified | Epidural | Epidural: 2% lidocaine with adrenaline 5 μg/ml of unspecified volume, followed by morphine 4 mg subsequent to delivery of placenta | I.M. diclofenac 75 mg, once only, on arrival to recovery | Not specified | I.M. pethidine |
| Diclofenac (20) | ||||||||||
| Luthman et al. 1994 [68] | Control (23) | International Journal of Obstetric Anesthesia | English | United Kingdom | Elective; surgical approach not specified | Spinal | Spinal: 2.75 ml 0.5% hyperbaric bupivacaine | P.R. diclofenac 100 mg, once only, at the end of surgery | I.V. morphine PCA | Not specified |
| Diclofenac (27) | ||||||||||
| Dennis et al. 1995 [69] | Control (25) | Anaesthesia | English | United Kingdom | Elective; surgical approach not specified | Spinal | Spinal: 2.5 ml 0.5% hyperbaric bupivacaine with morphine 200 μg | P.R. diclofenac 100 mg, once only, at the end of surgery | Not specified | P.O. paracetamol and dextropropoxyphene, and I.M. or I.V. morphine |
| Diclofenac (25) | ||||||||||
| Lee et al. 1997 [25] | Control (90) | Korean Journal of Anesthesiology | Korean | Korea | Not specified | General anesthesia | None | I.M. diclofenac 75 mg following incidence of postoperative pain and further doses every 12 h | I.V. pethidine or morphine PCA depending on group allocation | Not specified |
| Diclofenac (90) | ||||||||||
| Sia et al. 1997 [26] | Control (30) | Singapore Medical Journal | English | Singapore | Elective; surgical approach not specified | General anesthesia | Systemic: I.V. morphine 10 mg | P.R. diclofenac 100 mg, once only, following induction of general anesthesia and prior to surgical incision | I.V. morphine at a rate of 1.5 mg/h | I.M. pethidine |
| Diclofenac (30) | ||||||||||
| Kim et al. 1999 [27] | Control (40) | Korean Journal of Anesthesiology | Korean | Korea | Not specified | General anesthesia | None | I.M. diclofenac 75 mg following incidence of postoperative pain equal to or greater than 7 out of 10 and further doses every 12 h | I.V. pethidine or morphine PCA | Not specified |
| Diclofenac (40) | ||||||||||
| Lee et al. 1999 [28] | Control (30) | Korean Journal of Obstetrics and Gynecology | Korean | Korea | Not specified | General anesthesia | None | I.M. diclofenac 75 mg following incidence of postoperative pain equal to or greater than 7 out of 10 and further doses every 12 h | I.V. pethidine PCA | Not specified |
| Diclofenac (30) | ||||||||||
| Olofsson et al. 2000 [29] | Control (25) | European Journal of Obstetrics Gynecology and Reproductive Biology | English | Sweden | Elective; surgical approach not specified | Spinal | Spinal: 2.5 ml 0.5% hyperbaric bupivacaine | P.R. diclofenac 50 mg at the end of surgery and two further doses in the first 24 h | I.V. ketobemidone PCA | I.V. ketobemidone |
| Diclofenac (25) | ||||||||||
| Rashid et al. 2000 [30] | Control (20) | Saudi Medical Journal | English | Saudi Arabia | Elective or emergent; surgical approach not specified | General anesthesia | Not specified | P.R. diclofenac 100 mg at the end of surgery, 50 mg at 12 h, and 100 mg at 36 h | Not specified | I.M. pethidine |
| Diclofenac (20) | ||||||||||
| Al-Waili et al. 2001 [31] | Control (60) | Archives of Medical Research | English | United Arab Emirates | Elective | General anesthesia | Not specified | I.M. diclofenac 75 mg following incidence of postoperative pain and up to every 12 h thereafter | Not specified | I.M. pethidine |
| Diclofenac (60) | Surgical approach not specified | |||||||||
| Siddik et al. 2001 [32] | Control (40) | Regional Anesthesia and Pain Medicine | English | Lebanon | Elective; surgical approach not specified | Spinal | Spinal: 1.6 ml 0.75% hyperbaric bupivacaine with fentanyl 12.5 μg. | P.R. diclofenac 100 mg at the time of skin closure and further doses every 8 h in the first 24 h | I.V. propacetamol 2 g every 8 h in the first 24 h in only some groups. | I.V. morphine |
| Diclofenac (39) | Systemic: I.V. propacetamol in only some groups | I.V. morphine PCA | ||||||||
| Dahl et al. 2002 [33] | Control (42) | International Journal of Obstetric Anesthesia | English | Norway | Elective; surgical approach not specified | Spinal | Spinal: 2.2–2.4 ml 0.5% hyperbaric bupivacaine | P.R. diclofenac 100 mg on arrival to recovery, 12 h, and 24 h | P.O. paracetamol 1 g every 6 h | I.V. morphine |
| Diclofenac (40) | ||||||||||
| Wilder-Smith et al. 2003 [34] | Control (60) | Anesthesia and Analgesia | English | South Africa | Elective; surgical approach not specified | Spinal | Spinal: 1.8–2 ml 0.5% hyperbaric bupivacaine | I.M. diclofenac 75 mg following regression of sensory blockade to T10 and pain severity reported to be moderate | I.M. tramadol 100 mg as stat only in only some groups | I.V. morphine |
| Diclofenac (60) | ||||||||||
| Bourlert et al. 2005 [36] | Control (30) | Journal of the Medical Association of Thailand | English | Thailand | Not specified if elective or emergent; Pfannensteil approach | Not specified | Not specified | I.M. diclofenac 75 mg, once only, postoperatively | I.M. morphine 10 mg as stat dose. | Not specified |
| Diclofenac (34) | I.V. morphine PCA | |||||||||
| Munishankar et al. 2008 [37] | Control (24) | Anaesthesia | English | United Kingdom | Elective; surgical approach not specified | Spinal | Spinal: 2.25–2.5 ml 0.5% hyperbaric bupivacaine with fentanyl 12.5 μg | P.R. diclofenac 100 mg at the end of surgery followed by P.O. diclofenac 50 mg every 8 h | P.R. paracetamol 1 g. | I.V. morphine |
| Diclofenac (25) | P.O. paracetamol 1 g every 6 h. | |||||||||
| I.V. morphine PCA | ||||||||||
| Surakarn et al. 2009 [38] | Control (40) | Journal of the Medical Association of Thailand | English | Thailand | Elective; low midline approach | Spinal | Spinal: hyperbaric bupivacaine 10–12 mg with morphine 200 μg | I.M. diclofenac 75 mg within 2 h of the end of surgery and at 12 h | Not specified | I.M. tramadol |
| Diclofenac (40) | ||||||||||
| Thiengthong et al. 2012 [39] | Control (15) | Acta Anaesthesiologica Taiwanica | English | Thailand | Not specified if elective or emergent; Pfannensteil approach | Spinal | Spinal: 2.2–2.5 ml 0.5% hyperbaric bupivacaine with morphine 200 μg | I.V. diclofenac 75 mg, once only, at 12 h | None | I.V. tramadol |
| Diclofenac (14) | ||||||||||
| Adamou et al. 2014 [40] | Control (80) | Nigerian Medical Journal | English | Nigeria | Elective or emergent; surgical approach not specified | Neuraxial or general anesthesia | Determined by anesthesiologist | I.M. diclofenac 1 mg/kg following the end of surgery and every 12 h for 48 h | I.M. pentazocine 1 mg/kg every 6 h for 48 h | Not specified |
| Diclofenac (80) | ||||||||||
| Lotfalizade et al. 2015 [41] | Control (33) | Iranian Journal of Obstetrics, Gynecology and Infertility | Persian | Iran | Elective or emergent; surgical approach not specified | Spinal | Spinal: 2.4 ml 0.5% hyperbaric bupivacaine with adrenaline 200 μg and fentanyl 20 μg | P.R. diclofenac 100 mg of unspecified frequency following pain severity reported to be moderate | Not specified | |
| Diclofenac (33) | Tramadol 100 mg of unspecified route | |||||||||
| Olateju et al. 2016 [42] | Control (52) | Middle East Journal of Anesthesiology | English | Nigeria | Elective or emergent; low midline or Pfannensteil | Spinal | Spinal: 0.5% hyperbaric bupivacaine of unspecified volume | P.R. diclofenac 100 mg at the end of surgery, 12 h, and 24 h | I.M. pentazocine 30 mg every 6 h for 24 h | I.M. tramadol |
| Diclofenac (64) | ||||||||||
| Egede et al. 2017 [43] | Control (70) | Journal of Clinical and Diagnostic Research | English | Nigeria | Elective or emergent; Pfannensteil approach | Spinal | Not specified | I.M. diclofenac 75 mg within 1 h of the end of surgery and every 12 h for 24 h | I.M. pentazocine 30 mg every 4 h for 24 h | I.M. pentazocine |
| Diclofenac (70) | ||||||||||
| Kanta et al. 2021 [11] | Control (30) | Indian Journal of Anaesthesia | English | India | Elective or emergent; surgical approach not specified | Spinal | Spinal: 2.5 ml 0.5% hyperbaric bupivacaine with morphine 200 μg. | I.V. diclofenac 75 mg following delivery of neonate | Not specified | I.M. diclofenac |
| Diclofenac (30) | TAP block: 1.5 mg/kg 0.75% ropivacaine on each side | |||||||||
| Control vs diclofenac vs indomethacin | ||||||||||
| Akhavanakbari et al. 2013 [44] | Control (30) | Perspectives in Clinical Research | English | Iran | Not specified if elective or emergent; surgical approach not specified | Spinal | Spinal: 1.5–2 ml hyperbaric 5% lidocaine | P.R. diclofenac 50 mg or indomethacin 50 mg at the end of surgery and every 6 h for 24 h | Not specified | I.M. pethidine |
| Diclofenac (30) | ||||||||||
| Indomethacin (30) | ||||||||||
| Control vs diclofenac vs ketoprofen | ||||||||||
| Rorarius et al. 1993 [45] | Control (30) | British Journal of Anaesthesia | English | Finland | Elective; surgical approach not specified | Spinal or epidural | Spinal: 2.5–2.8 ml 0.5% hyperbaric bupivacaine. | I.V. diclofenac 150 mg or ketorolac 200 mg started at the end of surgery as an infusion over 24 h | Not specified | I.M. oxycodone |
| Diclofenac (29) | Epidural: Up to 20 ml 0.5% bupivacaine with or without 1% prilocaine if needed | |||||||||
| Ketoprofen (30) | ||||||||||
| Control vs ibuprofen vs ketorolac | ||||||||||
| Pagnoni et al. 1996 [47] | Control (32) | Clinical Drug Investigation | English | Italy | Not specified | General anesthesia | Systemic: I.V. fentanyl 0.1 μg/kg | I.M. ketorolac 30 mg or P.O. ibuprofen, once only, following incidence of postoperative pain equal to or greater than 60 out of 100 | Not specified | I.M. ketoprofen |
| Ibuprofen (30) | ||||||||||
| Ketorolac (30) | ||||||||||
| Control vs indomethacin | ||||||||||
| Pavy et al. 1995 [48] | Control (15) | Anaesthesia and Intensive Care | English | Australia | Elective; Pfannensteil approach | Spinal | Spinal: 1.2–1.4 ml 0.75% hyperbaric bupivacaine with fentanyl 10–15 μg and morphine 250–300 μg | P.R. indomethacin 200 mg at the end of surgery followed by P.R. indomethacin 100 mg every 12 h for 72 h | None | P.O. paracetamol and codeine, and parenteral opioids |
| Indomethacin (15) | ||||||||||
| Control vs ketorolac | ||||||||||
| Tzeng et al. 1994 [49] | Control (30) | Annals of the Academy of Medicine, Singapore | English | Taiwan, Republic of China | Elective; surgical approach not specified | Epidural | Epidural: 2% lidocaine with adrenaline 5 μg/ml of unspecified volume, followed by morphine 2 mg postoperatively | I.M. ketorolac 30 mg, once only, following incidence of postoperative pain | Not specified | I.M. pethidine |
| Ketorolac (30) | ||||||||||
| Cohen et al. 1996 [50] | Control (12) | International Journal of Obstetric Anesthesia | English | United States of America | Elective; Pfannensteil approach | Spinal | Spinal: 1.6 ml 0.75% hyperbaric bupivacaine and morphine 100 μg | I.V. ketorolac 60 mg 1 h after spinal anesthesia followed by I.V. ketorolac 30 mg every 6 h for three doses | Not specified | I.V. pethidine |
| Ketorolac (13) | Systemic: I.V. fentanyl 50–100 μg if needed | |||||||||
| Pavy et al. 2001 [51] | Control (20) | Anesthesia and Analgesia | English | Australia | Elective; surgical approach not specified | CSE | CSE: 2–2.5 0.5% hyperbaric bupivacaine with fentanyl 12.5 μg | I.V. ketorolac 15–30 mg after delivery of neonate followed by I.V. ketorolac 105–120 mg started in recovery as an infusion over 24 h | Patient-controlled epidural analgesia with bolus of pethidine 24 mg and lockout interval of 15 min | P.O. paracetamol and codeine |
| Ketorolac (24) | ||||||||||
| Lowder et al. 2003 [52] | Control (22) | American Journal of Obstetrics and Gynecology | English | United States of America | Not specified if elective or emergent; Pfannensteil approach | Neuraxial or general anesthesia | Determined by anesthesiologist | I.V. ketorolac 30 mg at the end of surgery and two further doses at unspecified time interval | I.V. hydromorphone, pethidine, or morphine PCA | Not specified |
| Ketorolac (22) | ||||||||||
| El-Tahan et al. 2007 [53] | Control (45) | International Journal of Obstetric Anesthesia | English | Egypt | Elective; Pfannensteil approach | General anesthesia | Systemic: I.V. fentanyl 1 μg/kg | I.V. ketorolac 15 mg before induction of general anesthesia followed by I.V. ketorolac as an infusion of 7.5 mg/hr until the end of surgery | None | I.V. tramadol |
| Ketorolac (45) | ||||||||||
| Khezri et al. 2018 [10] | Control (50) | Caspian Journal of Internal Medicine | English | Iran | Elective; surgical approach not specified | Spinal | Spinal: 2.5 ml 0.5% bupivacaine of unspecified baricity | I.V. ketorolac 30 mg, once only, before spinal anesthesia | Not specified | I.V. paracetamol |
| Ketorolac (50) | ||||||||||
| Control vs naproxen | ||||||||||
| Angle et al. 2002 [54] | Control (40) | Anesthesia and Analgesia | English | Canada | Elective | Spinal | Spinal: 1.2–1.8 ml 0.75% hyperbaric bupivacaine with fentanyl 10–20 μg and morphine 200 μg | P.R. naproxen 500 mg 1 h at the end of surgery followed by P.O. naproxen 550 mg every 12 h for 72 h | Not specified | P.O. paracetamol and codeine, and I.M. pethidine or morphine |
| Naproxen (40) | Pfannensteil approach | |||||||||
| Control vs parecoxib | ||||||||||
| Inthigood et al. 2017 [55] | Control (41) | Journal of Obstetrics and Gynaecology Research | English | Thailand | Elective; low midline or Pfannensteil approach | Spinal | Spinal: 2 ml 0.5% hyperbaric bupivacaine with morphine 200 μg | I.V. parecoxib 40 mg, once only, 2 h following the end of surgery | Not specified | I.V. pethidine |
| Parecoxib (41) | ||||||||||
| Control vs tenoxicam | ||||||||||
| Belzarena 1994 [56] | Control (40) | Regional Anesthesia | English | Brazil | Elective; surgical approach not specified | Spinal | Spinal: 3 ml 0.5% hyperbaric bupivacaine | I.V. tenoxicam 20 mg, once only, before spinal anesthesia | Not specified | Paracetamol and codeine of unspecified route |
| Tenoxicam (40) | ||||||||||
| Elhakim and Nafie 1995 [58] | Control (25) | British Journal of Anaesthesia | English | Egypt | Elective; surgical approach not specified | General anesthesia | Systemic: I.V. nalbuphine 0.25 mg/kg | I.V. tenoxicam 20 mg, once only, before induction of general anesthesia | None | I.M. nalbuphine |
| Tenoxicam (25) | ||||||||||
| Ro et al. 1997 [59] | Control (20) | Korean Journal of Anesthesiology | Korean | Korea | Not specified | General anesthesia | None | I.V. tenoxicam 0.3 mg/kg, once only, before induction of general anesthesia | I.V. morphine 0.1 mg/kg bolus followed by infusion of 0.015 mg/kg/h | Not specified |
| Tenoxicam (20) | ||||||||||
| Huang et al. 2002 [60] | Control (59) | Canadian Journal of Anaesthesia | English | Taiwan, Republic of China | Elective; surgical approach not specified | Spinal | Spinal: 1.8–2.2 ml 0.5% hyperbaric bupivacaine with morphine 150 μg | I.V. tenoxicam 40 mg, once only, following clamping of umbilical cord | Not specified | I.M. pethidine |
| Tenoxicam (58) | ||||||||||
| Hsu et al. 2003 [61] | Control (48) | Clinical Journal of Pain | English | Taiwan, Republic of China | Elective; surgical approach not specified | Spinal | Spinal: 12.5 mg bupivacaine of unspecified baricity, concentration, and volume | I.V. tenoxicam 20 mg, once only, following clamping of umbilical cord | I.V. morphine PCA | Not specified |
| Tenoxicam (45) | ||||||||||
| Yeh et al. 2005 [62] | Control (40) | Journal of the Formosan Medical Association | English | Taiwan, Republic of China | Elective; Pfannensteil approach | Spinal | Spinal: 1.8–2.2 ml 0.5% hyperbaric bupivacaine | I.V. tenoxicam 20 mg, once only, following clamping of umbilical cord | I.V. morphine PCA | Not specified |
| Tenoxicam (40) | ||||||||||
| Diclofenac vs ketoprofen | ||||||||||
| Hirahara et al. 2003 [63] | Diclofenac (22) | Revista Brasileira de Anestesiologia | English | Brazil | Not specified | Spinal | Spinal: 3 ml 0.5% hyperbaric bupivacaine with morphine 28 μg | I.M. diclofenac 75 mg or I.V. ketorolac 100 mg, once only, 90 min after spinal anesthesia | I.V. morphine PCA | Not specified |
| Ketoprofen (22) | ||||||||||
| Ketorolac vs parecoxib | ||||||||||
| Wong et al. 2010 [64] | Ketorolac (33) | Acta Anaesthesiologica Taiwanica | English | Taiwan, Republic of China | Elective; surgical approach not specified | Spinal | Not specified | I.V. parecoxib 40 mg in recovery room and two further doses at 24 h and 48 h, or I.V. ketorolac 30 mg in recovery room followed by I.V. ketorolac in morphine PCA, administered at a rate of 0.36 mg/h and patient-controlled bolus of 1.8 mg with an unspecified time interval | I.V. morphine PCA | I.V. morphine |
| Parecoxib (33) | ||||||||||
Table 2.
Network League Table for All the Interventions in regard to Cumulative Intravenous Morphine Equivalent Consumption at 24 h
Table 3.
Conclusion from the Results of the Analysis and GRADE Quality of Evidence Assessment for the Primary and Secondary Outcomes
| Outcome | Number of trials | Total number of participants | Number of direct comparisons | Number of indirect comparisons | MCID | Conclusions | Quality of evidence | Comments |
|---|---|---|---|---|---|---|---|---|
| Analgesia | ||||||||
| Pain score at rest at 8–12 h (0–100) [11,23,25,26,34,35,37,42,43,45,47–49,57,59,61–63] | 18 | 1,523 | 8 | 20 | 10 | Control clinically and statistically inferior to diclofenac and indomethacin | Low quality (⨁⨁) | Downgraded for serious limitations and imprecision |
| Indomethacin clinically and statistically superior to celecoxib | ||||||||
| No other statistical differences between interventions, but, with MCID of 10, clinical differences possible | ||||||||
| Pain score on movement at 8–12 h (0–100) [11,25,30,42,47,52,57] | 7 | 506 | 6 | 4 | 10 | Control statistically inferior but clinically equivalent to diclofenac | Low quality (⨁⨁) | Downgraded for serious limitations and imprecision |
| No statistical differences between interventions, but, with MCID of 10, clinical differences possible | ||||||||
| Pain score at rest at 24 h (0–100) [11,23,25,34,37,38,41–45,47–49,56,59,61–65,67] | 22 | 1,790 | 9 | 19 | 10 | Control clinically and statistically inferior to diclofenac | Low quality (⨁⨁) | Downgraded for serious limitations and imprecision |
| Control statistically inferior but clinically equivalent to tenoxicam | ||||||||
| No other statistical differences between interventions, but, with MCID of 10, clinical differences possible | ||||||||
| Pain score on movement at 24 h (0–100) [11,25,30,42,44,47,52,55,64] | 9 | 582 | 5 | 10 | 10 | Control clinically and statistically inferior to diclofenac | Low quality (⨁⨁) | Downgraded for serious limitations and imprecision |
| No other statistical differences between interventions, but, with MCID of 10, clinical differences possible | ||||||||
| Pain score at rest at 48 h (0–100) [25,34,37,43,45,62] | 6 | 571 | 3 | 3 | 10 | No statistical differences between interventions, but, with MCID of 10, clinical differences possible | Low quality (⨁⨁) | Downgraded for serious limitations and imprecision |
| Pain score on movement at 48 h (0–100) [25,30,52,55] | 4 | 235 | 4 | 6 | 10 | Control clinically and statistically inferior to indomethacin | Low quality (⨁⨁) | Downgraded for serious limitations and imprecision |
| No clinical or statistical difference between control and celecoxib + parecoxib | ||||||||
| Indomethacin clinically and statistically superior to celecoxib + parecoxib, diclofenac and ketorolac | ||||||||
| No other statistical differences between interventions, but, with MCID of 10, clinical differences possible | ||||||||
| Need for rescue analgesia (%) [23,25–28,32,37,40,43,47,51,53–55,57–60,63,66] | 20 | 1,586 | 10 | 35 | 20% | Control statistically and clinically inferior to diclofenac, ketoprofen and tenoxicam | Very low quality (⨁) | Downgraded for serious limitations, imprecision, and publication bias |
| Ketoprofen statistically and clinically superior to celecoxib + parecoxib | ||||||||
| No other statistical differences between interventions, but, with MCID of 20%, clinical differences possible | ||||||||
| Time to rescue analgesia (min) [10,11,24,30,40,46,49–52,54,55,57,58,60] | 15 | 1,076 | 10 | 18 | 60 min | Control clinically and statistically inferior to diclofenac, ketorolac and naproxen | Very low quality (⨁) | Downgraded for serious limitations, imprecision, inconsistency, and publication bias |
| Diclofenac, ibuprofen, indomethacin and ketorolac clinically and statistically superior to celecoxib | ||||||||
| No other statistical differences between interventions, but, with MCID of 60 min, clinical differences possible | ||||||||
| Cumulative intravenous morphine equivalent consumption at 8–12 h (mg) [26,33–35,39,55] | 6 | 364 | 2 | 1 | 10 mg | Control clinically and statistically inferior to diclofenac | Low quality (⨁⨁) | Downgraded for serious limitations and imprecision |
| No other statistical differences between interventions, but, with MCID of 10 mg, clinical differences possible | ||||||||
| Cumulative intravenous morphine equivalent consumption at 24 h (mg) [24,33–35,38–40,42,44,49,50,54,56,59,61,64,65,67] | 18 | 1,228 | 9 | 12 | 10 mg | Control clinically and statistically inferior to diclofenac, indomethacin, ketorolac and tenoxicam | Very low quality (⨁) | Downgraded for serious limitations, imprecision and publication bias |
| No other statistical differences between interventions, but, with MCID of 10 mg, clinical differences possible | ||||||||
| Cumulative intravenous morphine equivalent consumption at 48 h (mg) [31,33,34] | 3 | 320 | - | - | 10 mg | Pairwise comparison only | Moderate quality (⨁⨁⨁) | Downgraded for serious limitations |
| Control clinically and statistically inferior to diclofenac (MD: -46.29, 95% CI [-60.71, -31.86], I2 = 73%; P < 0.0001) | ||||||||
| Cumulative in-hospital intravenous morphine equivalent consumption (mg) [29,31,41,55,67] | 5 | 404 | 3 | 3 | 10 mg | Control clinically and statistically inferior to diclofenac, ketorolac and parecoxib | Low quality (⨁⨁) | Downgraded for serious limitations and imprecision |
| No other statistical differences between interventions, but, with MCID of 10 mg, clinical differences possible | ||||||||
| Side effects | ||||||||
| Rate of postoperative nausea and/or vomiting at 24 h (%) [10,29,36,39–41,44,48,53,55,61,64,67] | 13 | 938 | 4 | 6 | 20% | No statistical differences between interventions, but, with MCID of 20%, clinical differences possible | Low quality (⨁⨁) | Downgraded for serious limitations and imprecision |
| Rate of postoperative nausea and/or vomiting at 48 h (%) [52,55] | 2 | 74 | 2 | 1 | 20% | No statistical differences between interventions, but, with MCID of 20%, clinical differences possible | Very low quality (⨁) | Downgraded for serious limitations, imprecision and publication bias |
| Rate of in-hospital postoperative nausea and/or vomiting (%) [27,28,30–34,38,42,45,47,54,57,60,62,63,66] | 17 | 1,387 | 4 | 6 | 20% | No statistical differences between interventions, but, with MCID of 20%, clinical differences possible | Low quality (⨁⨁) | Downgraded for serious limitations and imprecision |
| Rate of pruritus at 24 h (%) [52,53,55,64,67] | 5 | 293 | 4 | 6 | 20% | No statistical differences between interventions, but, with MCID of 20%, clinical differences possible | Low quality (⨁⨁) | Downgraded for serious limitations and imprecision |
| Rate of pruritus at 48 h (%) [52,55] | 2 | 74 | 2 | 1 | 20% | No statistical differences between interventions, but, with MCID of 20%, clinical differences possible | Very low quality (⨁) | Downgraded for serious limitations, imprecision and publication bias |
| Rate of in-hospital pruritus (%) [23,25,27,28,30,31,34,36,38,54,60,62,63,66] | 14 | 1,043 | 6 | 15 | 20% | Ketoprofen statistically and clinically superior to celecoxib + parecoxib | Low quality (⨁⨁) | Downgraded for serious limitations and imprecision |
| No other statistical differences between interventions, but, with MCID of 20%, clinical differences possible | ||||||||
| Rate of sedation at 24 h (%) [29,36,37,40,48,55,61,67] | 8 | 630 | 4 | 6 | 20% | Control statistically and clinically inferior to diclofenac | Low quality (⨁⨁) | Downgraded for serious limitations and imprecision |
| No other statistical differences between interventions, but, with MCID of 20%, clinical differences possible | ||||||||
| Rate of sedation at 48 h (%) [55] | 1 | 44 | - | - | 20% | In one trial, no statistical difference between control and ketorolac | - | - |
| Rate of in-hospital sedation (%) [31,33,34,38,45] | 5 | 559 | - | - | 20% | Pairwise comparison only | Moderate quality (⨁⨁⨁) | Downgraded for serious limitations |
| Control clinically and statistically inferior to diclofenac (RR: 0.43, 95% CI [0.26, 0.73], I2 = 13; P = 0.002) | ||||||||
| Functional outcomes | ||||||||
| Hospital length of stay (h) [39,44,48,67] | 4 | 317 | - | - | 6 h | Pairwise comparison | Moderate quality (⨁⨁⨁) | Downgraded for serious limitations |
| Control statistically inferior but clinically equivalent to diclofenac (MD: -0.48, 95% CI [-0.88, -0.08], I2 = 0%; P = 0.02) | ||||||||
| In one trial, no statistical difference between ketorolac and parecoxib | ||||||||
References
1. Karlström A, Engström-Olofsson R, Norbergh KG, Sjöling M, Hildingsson I. Postoperative pain after cesarean birth affects breastfeeding and infant care. J Obstet Gynecol Neonatal Nurs 2007; 36: 430-40.


2. Gerbershagen HJ, Aduckathil S, van Wijck AJ, Peelen LM, Kalkman CJ, Meissner W. Pain intensity on the first day after surgery: a prospective cohort study comparing 179 surgical procedures. Anesthesiology 2013; 118: 934-44.


3. Carvalho B, Cohen SE, Lipman SS, Fuller A, Mathusamy AD, Macario A. Patient preferences for anesthesia outcomes associated with cesarean delivery. Anesth Analg 2005; 101: 1182-7.


4. Yurashevich M, Carvalho B, Butwick AJ, Ando K, Flood PD. Determinants of women's dissatisfaction with anaesthesia care in labour and delivery. Anaesthesia 2019; 74: 1112-20.



5. Eisenach JC, Pan PH, Smiley R, Lavand'homme P, Landau R, Houle TT. Severity of acute pain after childbirth, but not type of delivery, predicts persistent pain and postpartum depression. Pain 2008; 140: 87-94.



6. Kainu JP, Halmesmäki E, Korttila KT, Sarvela PJ. Persistent pain after cesarean delivery and vaginal delivery: a prospective cohort study. Anesth Analg 2016; 123: 1535-45.


7. Burian M, Geisslinger G. COX-dependent mechanisms involved in the antinociceptive action of NSAIDs at central and peripheral sites. Pharmacol Ther 2005; 107: 139-54.


8. Zeng AM, Nami NF, Wu CL, Murphy JD. The analgesic efficacy of nonsteroidal anti-inflammatory agents (NSAIDs) in patients undergoing cesarean deliveries: a meta-analysis. Reg Anesth Pain Med 2016; 41: 763-72.


9. Roofthooft E, Joshi GP, Rawal N, Van de Velde M. PROSPECT guideline for elective caesarean section: updated systematic review and procedure-specific postoperative pain management recommendations. Anaesthesia 2021; 76: 665-80.




10. Khezri MB, Mosallaei MA, Ebtehaj M, Mohammadi N. Comparison of preemptive effect of intravenous ketorolac versus meperidine on postoperative shivering and pain in patients undergoing cesarean section under spinal anesthesia: a prospective, randomized, double-blind study. Caspian J Intern Med 2018; 9: 151-7.
11. Kanta B, Sonali D, Gazala P, Yunus K, Kiran K. A randomised comparative study of transversus abdominis plane block with or without intravenous diclofenac sodium as a component of multimodal regimen for post-operative analgesia following caesarean section. Indian J Anaesth 2021; 65: 316-20.



12. Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 2015; 162: 777-84.


13. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev 2016; 5: 210.




14. Shim H, Gan TJ. Side effect profiles of different opioids in the perioperative setting: are they different and can we reduce them? Br J Anaesth 2019; 123: 266-8.


15. Laigaard J, Pedersen C, Rønsbo TN, Mathiesen O, Karlsen AP. Minimal clinically important differences in randomised clinical trials on pain management after total hip and knee arthroplasty: a systematic review. Br J Anaesth 2021; 126: 1029-37.


16. Kleif J, Waage J, Christensen KB, Gögenur I. Systematic review of the QoR-15 score, a patient- reported outcome measure measuring quality of recovery after surgery and anaesthesia. Br J Anaesth 2018; 120: 28-36.


17. Cochrane Handbook for Systematic Reviews of Interventions. 2nd Edition. Edited by Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al.: Chichester (UK), John Wiley & Sons. 2019.
18. National Institute for Health and Care Excellence. Prescribing in Palliative Care. British National Formulary 80th Ed. Joint Formulary Committee [Internet]. Manchester: NICE [cited 2021 July 1]. Available from https://bnf.nice.org.uk/medicines-guidance/prescribing-in-palliative-care/
19. Faculty of Pain Medicine of the Royal College of Anaesthetists. Dose equivalents and changing opioids [Internet]. London: FPM [cited 2021 July 1]. Available from https://fpm.ac.uk/opioids-aware-structured-approach-opioid-prescribing/dose-equivalents-and-changing-opioids
20. Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta-analysis in STATA. PLoS One 2013; 8: e76654.



21. Chaimani A, Salanti G. Visualizing assumptions and results in network meta-analysis: the network graphs package. Stata J 2015; 15: 905-50.


22. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21: 1539-58.


23. Salanti G, Del Giovane C, Chaimani A, Caldwell DM, Higgins JP. Evaluating the quality of evidence from a network meta-analysis. PLoS One 2014; 9: e99682.



24. Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019; 366: l4898.

25. Lee BH, Lim YG, Chea JS, Kim CJ, Chung MY, Jung JY. Morphine and meperidine analgesic effect using intravenous PCA of intramuscular diclofenac after cesarean section. Korean J Anesthesiol 1997; 33: 510-6.

26. Sia AT, Thomas E, Chong JL, Loo CC. Combination of suppository diclofenac and intravenous morphine infusion in post-caesarean section pain relief--a step towards balanced analgesia? Singapore Med J 1997; 38: 68-70.

27. Kim CJ, Chea JS, Chung MY, Lee BH, Yoon JW. The analgesic and hemostatic effects of diclofenac as adjuvant of intravenous opioid using PCA after cesarean section. Korean J Anesthesiol 1999; 36: 256-62.

28. Lee Y, Kim SJ, Kwun I, Chung DY, Kim CY, Kim SP, et al. The influence of choice of pain control method on analgesic effect and postoperative progress after cesarean section. Korean J Obstet Gynecol 1999; 42: 2513-8.

29. Olofsson CI, Legeby MH, Nygårds EB, Ostman KM. Diclofenac in the treatment of pain after caesarean delivery. An opioid-saving strategy. Eur J Obstet Gynecol Reprod Biol 2000; 88: 143-6.


30. Rashid M, Jaruidi HM. The use of rectal diclofenac for post-cesarean analgesia. Saudi Med J 2000; 21: 145-9.

31. Al-Waili NS. Efficacy and safety of repeated postoperative administration of intramuscular diclofenac sodium in the treatment of post-cesarean section pain: a double-blind study. Arch Med Res 2001; 32: 148-54.


32. Siddik SM, Aouad MT, Jalbout MI, Rizk LB, Kamar GH, Baraka AS. Diclofenac and/or propacetamol for postoperative pain management after cesarean delivery in patients receiving patient controlled analgesia morphine. Reg Anesth Pain Med 2001; 26: 310-5.


33. Dahl V, Hagen IE, Sveen AM, Norseng H, Koss KS, Steen T. High-dose diclofenac for postoperative analgesia after elective caesarean section in regional anaesthesia. Int J Obstet Anesth 2002; 11: 91-4.


34. Wilder-Smith CH, Hill L, Dyer RA, Torr G, Coetzee E. Postoperative sensitization and pain after cesarean delivery and the effects of single im doses of tramadol and diclofenac alone and in combination. Anesth Analg 2003; 97: 526-33.


35. Lee LH, Irwin MG, Lim J, Wong CK. The effect of celecoxib on intrathecal morphine-induced pruritus in patients undergoing Caesarean section. Anaesthesia 2004; 59: 876-80.


36. Bourlert A. Diclofenac intramuscular single dose to decrease pain in post operative Caesarean section: a double blind randomized controlled trial. J Med Assoc Thai 2005; 88: 15-9.
37. Munishankar B, Fettes P, Moore C, McLeod GA. A double-blind randomised controlled trial of paracetamol, diclofenac or the combination for pain relief after caesarean section. Int J Obstet Anesth 2008; 17: 9-14.


38. Surakarn J, Tannirandorn Y. Intramuscular diclofenac for analgesia after cesarean delivery: a randomized controlled trial. J Med Assoc Thai 2009; 92: 733-7.
39. Thienthong S, Chongsomchai C, Kemthong W. A placebo-controlled, double-blind, randomized study of single-dose intravenous diclofenac for pain relief after a cesarean section. Acta Anaesthesiol Taiwan 2012; 50: 150-2.

40. Adamou N, Tukur J, Muhammad Z, Galadanci H. A randomised controlled trial of opioid only versus combined opioid and non-steroidal anti inflammatory analgesics for pain relief in the first 48 hours after Caesarean section. Niger Med J 2014; 55: 369-73.



41. Lotfalizade M, Zirak N, Ghomian N, Ebrahimi F, Mohammadnejad M. Comparison of the effects of diclofenac suppository and tramadol injection and the combination of these two drugs on pain after spinal anesthesia for cesarean. Iran J Obstet Gynecol Infertil 2015; 17: 1-5.

42. Olateju SO, Adenekan AT, Olufolabi AJ, Owojuyigbe AM, Adetoye AO, Ajenifuja KO, et al. Pentazocine versus pentazocine with rectal diclofenac for postoperative pain relief after cesarean section- a double blind randomized placebo controlled trial in a low resource area. Middle East J Anaesthesiol 2016; 23: 443-8.

43. Egede JO, Ajah LO, Umeora OU, Ozumba BC, Onoh RC, Obuna JA, et al. Pentazocine alone versus pentazocine plus diclofenac for pain relief in the first 24 hours after caesarean section: a randomized controlled study. J Clin Diagn Res 2017; 11: QC01-5.



44. Akhavanakbari G, Entezariasl M, Isazadehfar K, Kahnamoyiagdam F. The effects of indomethacin, diclofenac, and acetaminophen suppository on pain and opioids consumption after cesarean section. Perspect Clin Res 2013; 4: 136-41.



45. Rorarius MG, Suominen P, Baer GA, Romppanen O, Tuimala R. Diclofenac and ketoprofen for pain treatment after elective caesarean section. Br J Anaesth 1993; 70: 293-7.


46. Fong WP, Yang LC, Wu JI, Chen HS, Tan PH. Does celecoxib have pre-emptive analgesic effect after Caesarean section surgery? Br J Anaesth 2008; 100: 861-2.


47. Pagnoni B, Vignali M, Colella S, Monopoli R, Tiengo M. Comparative efficacy of oral ibuprofen arginine and intramuscular ketorolac in patients with postcaesarean section pain. Clin Drug Investig 1996; 11: 15-21.

48. Pavy TJ, Gambling DR, Merrick PM, Douglas MJ. Rectal indomethacin potentiates spinal morphine analgesia after caesarean delivery. Anaesth Intensive Care 1995; 23: 555-9.



49. Tzeng JI, Mok MS. Combination of intramuscular Ketorolac and low dose epidural morphine for the relief of post-caesarean pain. Ann Acad Med Singap 1994; 23(6 Suppl): 10-3.
50. Cohen SE, Desai JB, Ratner EF, Riley ET, Halpern J. Ketorolac and spinal morphine for postcesarean analgesia. Int J Obstet Anesth 1996; 5: 14-8.


51. Pavy TJ, Paech MJ, Evans SF. The effect of intravenous ketorolac on opioid requirement and pain after cesarean delivery. Anesth Analg 2001; 92: 1010-4.


52. Lowder JL, Shackelford DP, Holbert D, Beste TM. A randomized, controlled trial to compare ketorolac tromethamine versus placebo after cesarean section to reduce pain and narcotic usage. Am J Obstet Gynecol 2003; 189: 1559-62.


53. El-Tahan MR, Warda OM, Yasseen AM, Attallah MM, Matter MK. A randomized study of the effects of preoperative ketorolac on general anaesthesia for caesarean section. Int J Obstet Anesth 2007; 16: 214-20. Erratum in: Int J Obstet Anesth 2023; 56: 103941.


54. Angle PJ, Halpern SH, Leighton BL, Szalai JP, Gnanendran K, Kronberg JE. A randomized controlled trial examining the effect of naproxen on analgesia during the second day after cesarean delivery. Anesth Analg 2002; 95: 741-5.


55. Inthigood N, Lertbunnaphong T, Jaishuen A. Efficacy of a single 40-mg intravenous dose of parecoxib for postoperative pain control after elective cesarean delivery: a double-blind randomized placebo-controlled trial. J Obstet Gynaecol Res 2017; 43: 92-9.



56. Belzarena SD. Evaluation of intravenous tenoxicam for postoperative cesarean delivery pain relief. Preliminary report. Reg Anesth 1994; 19: 408-11.

57. Paech MJ, McDonnell NJ, Sinha A, Baber C, Nathan EA. A randomised controlled trial of parecoxib, celecoxib and paracetamol as adjuncts to patient-controlled epidural analgesia after caesarean delivery. Anaesth Intensive Care 2014; 42: 15-22.



58. Elhakim M, Nafie M. I.v. tenoxicam for analgesia during caesarean section. Br J Anaesth 1995; 74: 643-6.


59. Ro MS, Do GH, Kim JH, Gang HS. Incomplete preemptive analgesic effects of tenoxicam on continuous intravenous analgesia with morphine after cesarean section. Korean J Anesthesiol 1997; 33: 1154-58.

60. Huang YC, Tsai SK, Huang CH, Wang MH, Lin PL, Chen LK, et al. Intravenous tenoxicam reduces uterine cramps after Cesarean delivery. Can J Anaesth 2002; 49: 384-7.



61. Hsu HW, Cheng YJ, Chen LK, Wang YP, Lin CJ, Lee CN, et al. Differential analgesic effect of tenoxicam on the wound pain and uterine cramping pain after cesarean section. Clin J Pain 2003; 19: 55-8.


62. Yeh YC, Chen SY, Lin CJ, Yeh HM, Sun WZ. Differential analgesic effect of tenoxicam on post-cesarean uterine cramping pain between primiparous and multiparous women. J Formos Med Assoc 2005; 104: 647-51.

63. Hirahara JT, Bliacheriene S, Yamaguchi ET, Rosa MC, Cardoso MM. Post-cesarean section analgesia with low spinal morphine doses and systemic nonsteroidal anti-inflammatory drug: diclofenac versus ketoprofen. Rev Bras Anestesiol 2003; 53: 737-42.


64. Wong JO, Tan TD, Cheu NW, Wang YR, Liao CH, Chuang FH, et al. Comparison of the efficacy of parecoxib versus ketorolac combined with morphine on patient-controlled analgesia for post-cesarean delivery pain management. Acta Anaesthesiol Taiwan 2010; 48: 174-7.

65. Bush DJ, Lyons G, MacDonald R. Diclofenac for analgesia after caesarean section. Anaesthesia 1992; 47: 1075-7.


66. Sun HL, Wu CC, Lin MS, Chang CF, Mok MS. Combination of low-dose epidural morphine and intramuscular diclofenac sodium in postcesarean analgesia. Anesth Analg 1992; 75: 64-8.


67. Sun HL, Wu CC, Lin MS, Chang CF. Effects of epidural morphine and intramuscular diclofenac combination in postcesarean analgesia: a dose-range study. Anesth Analg 1993; 76: 284-8.

68. Luthman J, Kay NH, White JB. The morphine sparing effect of diclofenac sodium following caesarean section under spinal anaesthesia. Int J Obstet Anesth 1994; 3: 82-6.


69. Dennis AR, Leeson-Payne CG, Hobbs GJ. Analgesia after caesarean section. The use of rectal diclofenac as an adjunct to spinal morphine. Anaesthesia 1995; 50: 297-9.


71. Bhala N, Emberson J, Merhi A, Abramson S, Arber N, Baron JA, et al. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet 2013; 382: 769-79.



72. Brune K, Patrignani P. New insights into the use of currently available non-steroidal anti-inflammatory drugs. J Pain Res 2015; 8: 105-18.



73. Brune K, Renner B, Hinz B. Using pharmacokinetic principles to optimize pain therapy. Nat Rev Rheumatol 2010; 6: 589-98.



74. Laprairie RB, Mohamed KA, Zagzoog A, Kelly ME, Stevenson LA, Pertwee R, et al. Indomethacin enhances type 1 cannabinoid receptor signaling. Front Mol Neurosci 2019; 12: 257.



75. Vane JR, Botting RM. Mechanism of action of nonsteroidal anti-inflammatory drugs. Am J Med 1998; 104: S2-8.

76. Wang R, Dasgupta A, Ward MM. Comparative efficacy of non-steroidal anti-inflammatory drugs in ankylosing spondylitis: a Bayesian network meta-analysis of clinical trials. Ann Rheum Dis 2016; 75: 1152-60.


77. da Costa BR, Reichenbach S, Keller N, Nartey L, Wandel S, Jüni P, et al. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis. Lancet 2017; 390: e21-33.


78. Paglia MD, Silva MT, Lopes LC, Barberato-Filho S, Mazzei LG, Abe FC, et al. Use of corticoids and non-steroidal anti-inflammatories in the treatment of rheumatoid arthritis: systematic review and network meta-analysis. PLoS One 2021; 16: e0248866.



79. Ong CK, Seymour RA, Lirk P, Merry AF. Combining paracetamol (acetaminophen) with nonsteroidal antiinflammatory drugs: a qualitative systematic review of analgesic efficacy for acute postoperative pain. Anesth Analg 2010; 110: 1170-9.


80. Sutton CD, Carvalho B. Optimal pain management after cesarean delivery. Anesthesiol Clin 2017; 35: 107-24.


81. Myles PS, Myles DB, Galagher W, Boyd D, Chew C, MacDonald N, et al. Measuring acute postoperative pain using the visual analog scale: the minimal clinically important difference and patient acceptable symptom state. Br J Anaesth 2017; 118: 424-9.


82. Olsen MF, Bjerre E, Hansen MD, Tendal B, Hilden J, Hróbjartsson A. Minimum clinically important differences in chronic pain vary considerably by baseline pain and methodological factors: systematic review of empirical studies. J Clin Epidemiol 2018; 101: 87-106.e2.












