|
 |
| Korean J Anesthesiol > Volume 72(1); 2019 > Article |
|
Abstract
Background
Postoperative nausea and vomiting (PONV) is a common complication following laparascopic surgery. This study compared the effect of intraperitoneal versus intravenous dexamethasone for reducing PONV after gynecological laparoscopic surgeries.
Methods
Eighty adult female patients, American Society of Anesthesiologists physical status I–II, scheduled for gynecological laparoscopic surgery were randomized to receive 8 mg dexamethasone intravenously (IV) (n = 40) or intraperitoneally (IP) (n = 40). The primary outcome was the PONV incidence during the first 24 h after laparoscopy. Secondary outcomes included visual analogue scale (VAS) pain scores, total rescue analgesic consumption during the first 24 h postoperatively, the need for rescue antiemetic drugs, and the incidence of complications that may accompany these medications.
Results
Eleven women (27.5%) in the IV group, versus only 3 (7.5%) women in the IP group, experienced nausea during the first 24 h postlaparoscopy (P = 0.037). However, 5 patients (12.5%) in the IV group, versus only 2 patients (5.0%) in the IP group, experienced vomiting (P = 0.424). No statistically significant differences were seen in the severity of nausea or the need for rescue antiemetics. The IV group had a higher rate of side-effects than the IP group (27.5% vs. 7.5%, P = 0.037). Headache and dizziness were common side effects in the IV dexamethasone group. The groups did not differ significantly in terms of mean VAS score for pain and total meperidine consumption during the first 24 h postoperatively.
Postoperative nausea and vomiting (PONV) is a common complication after general anesthesia [1], with an overall incidence up to 30% in all surgeries [2,3]. Several factors increase the risk for PONV, such as female sex, a history of previous PONV, history of motion sickness, non-smoking status, use of opioids, and a long duration of surgery [4,5].
Many studies have demonstrated the role of dexamethasone in preventing PONV in various surgical procedures, such as laparoscopic cholecystectomy [6], laparoscopic tubal ligation [7], hysterectomy [8], and thyroidectomy [9].
In most previous studies, dexamethasone was administered via an intravenous route with different doses [10]. Despite the evident benefit of dexamethasone in preventing PONV, a single injected dose might produce a wide range of short- and longterm side-effects, such as perioperative hyperglycemia and increased risk of infection [11,12]. A single dose of dexamethasone injected into the peritoneal cavity has been reported to relieve the pain after gynecological laparoscopic surgery [13]. To our knowledge, no previous studies in the literature have compared the effect of intraperitoneal versus intravenous dexamethasone in PONV. We hypothesized that direct injection of dexamethasone intraperitoneally would reduce the incidence of PONV after gynecological laparoscopic surgeries.
Therefore, the aim of this study was to compare the efficacy of intravenous versus intraperitoneal administration of dexamethasone in reducing the incidence of PONV after gynecological laparoscopic surgeries.
The primary outcome was the incidence of PONV during the first 24 h after laparoscopy. Secondary outcomes included visual analogue scale (VAS) pain scores, total consumption of rescue analgesic during the first 24 h postoperatively, the need for rescue antiemetic drugs, and the incidence of delayed complications that may accompany medications within the first week postoperatively.
This prospective, randomized, double-blind clinical trial was conducted in Assiut university hospital, Assiut, Egypt between the 1st of November 2016 and the 30th of September 2017. The institutional research ethics review board approved the study. This trial was registered at ClinicalTrials.gov (NCT02947672).
All women scheduled for gynecological laparoscopic surgery were invited to participate. All eligible participants included in the study signed written informed consent before participation, after receiving an explanation of the nature of the study. Women aged 18–40 years old, with American Society of Anesthesiologists physical status I–II were included.
We excluded pregnant or lactating women, patients with coagulopathy or under anticoagulant therapy, gastrointestinal diseases, motion sickness, diabetes mellitus, neurological or neuromuscular disorders, renal impairment, or hepatic dysfunction. Additionally, women with a body mass index > 40 kg/m2 or who received antiemetic or systemic steroids within 24 h before surgery, were also excluded.
Sample size calculation was based on the study of Asadollah et al. [14] who reported that the incidence of PONV in patients undergoing gynecological laparoscopy and who received IV dexamethasone was 39.9%. We considered a reduction of the incidence of PONV to 10% in the first 24 h postlaparoscopy as significant. Considering an alpha error of 0.05 and a statistical power of 80%, a sample size of at least 39 women in each group would be required (Odds ratio = 0.17).
Participants were randomized in a 1 : 1 ratio to receive intravenous or intraperitoneal dexamethasone. Randomization was based on computer-generated codes maintained in sequentially numbered opaque envelopes. Each envelope had a card noting the intervention type inside.
Patients were randomly allocated into 2 groups: the intravenous group (IV group) (n = 40), who received 8 mg IV dexamethasone in 2 ml (DexamethasoneⓇ; Amryia Pharm, Egypt) at the end of the procedure, before the withdrawal of the laparoscopic trocar, in addition to 2 ml of preservative-free 0.9% saline intraperitoneally. The intraperitoneal group (IP group) (n = 40) received the same dose of dexamethasone intraperitoneally at the end of the procedure, before laparoscopic trocar withdrawal, in addition to 2 ml of preservative-free 0.9% saline intravenously. All patients, investigators collecting the postoperative data, and nurses involved in the postoperative care of patients were blinded to the randomization.
One of the study investigators approached the scheduled patients and collected their baseline data: age, weight, height, and Apfel risk score assessment [4]. The duration of anesthesia was calculated from the injection of fentanyl until reversal of anesthesia and complete recovery of the patient. The duration of surgery was calculated from the time of umbilical incision for trocar insertion until placement of the final stitch to suture the laparoscopic incisions.
All patients fasted from midnight and were premedicated with midazolam 2 mg before arriving at the operating theater. Heart rate, non-invasive blood pressure, pulse oximetry, electrocardiography, and end-tidal carbon dioxide levels were recorded before surgery, and then every 15 min during surgery. The anesthetic technique was standardized for all patients, with anesthesia being induced with fentanyl 1.0 μg/kg and propofol 2–2.5 mg/kg. Then, 0.6 mg/kg rocuronium was administered to facilitate endotracheal intubation. Anesthesia was maintained with 2%–3% isoflurane in 50% oxygen/air. Volume-controlled ventilation was instituted and both tidal volume and respiratory rates were adjusted to maintain the end-tidal CO2 at around 35 mmHg. If the heart rate or mean blood pressure increased by > 20%, IV fentanyl 0.5 μg/kg was administered. The total intraoperative fentanyl requirement was recorded. Additional rocuronium (5–10 mg) was administered if clinically required to maintain an intraoperative train-of-four count of 2–3. An orogastric tube was inserted to empty the stomach of air. During laparoscopy, the peritoneal cavity was insufflated with carbon dioxide to keep intra-abdominal pressure < 14 mmHg. After gas deflation, all patients received 1 g paracetamol infusion over a period of 15 min. At the end of the procedure, anesthesia was reversed using IV neostigmine 2.5 mg plus atropine 1 mg to start spontaneous breathing. Patients were extubated when they fulfilled the criteria for extubation as adequate oxygenation and ventilation, hemodynamic stability and full reversal of muscle relaxants. The surgeons and anesthesiologists who managed the patients were blinded to the group assignment. All patients were transferred to the post-anesthesia care unit for post-operative follow-up. Follow-up was performed every 6 h for 24 h postoperatively. Each patient was asked about the occurrence of nausea, retching, and vomiting [15].
During the preoperative visit, all patients were familiarized with a VAS of 0–100 mm for PONV [7]. On this scale, a score of 0 meant no nausea, while a score of 100 meant the worst imaginable nausea. Occurrence of vomiting or retching was scored as 100. When moderate or severe nausea (VAS score > 40) or vomiting was present, ondansetron 4 mg, IV, was administered slowly as a rescue antiemetic.
VAS pain score (no pain = 0, worst possible pain = 10) was used to assess postoperative pain. A nurse blinded to the patient allocation group evaluated the pain scores on arrival in the PACU and at 1, 4, 8, 12, and 24 h postoperatively. The 24 h aggregate pain scores were calculated. Intramuscular meperidine (1 mg/kg) was administered upon patient request or when the VAS score of pain was > 3. The total consumption of rescue analgesic during the first 24 h postoperatively was recorded. All women were asked to report any possible side effects of study drug, such as dizziness, headache, neck/shoulder pain, or fainting during the first 24 h postoperatively, or delayed complications, such as wound infection or delay in wound healing during the first week postoperatively.
All data were analyzed using SPSS software (version 21, USA). Qualitative data were expressed as frequency and percentage. Fisher’s exact test was used to examine the relation between qualitative variables. Quantitative data were presented in terms of mean and SD and compared using Student’s t-test or the Mann–Whitney U test according to the normality of distribution (mean ± SD or median IQR as appropriate). Normality of data distribution was assessed with the Shapiro–Wilkes test. P values < 0.05 were considered statistically significant.
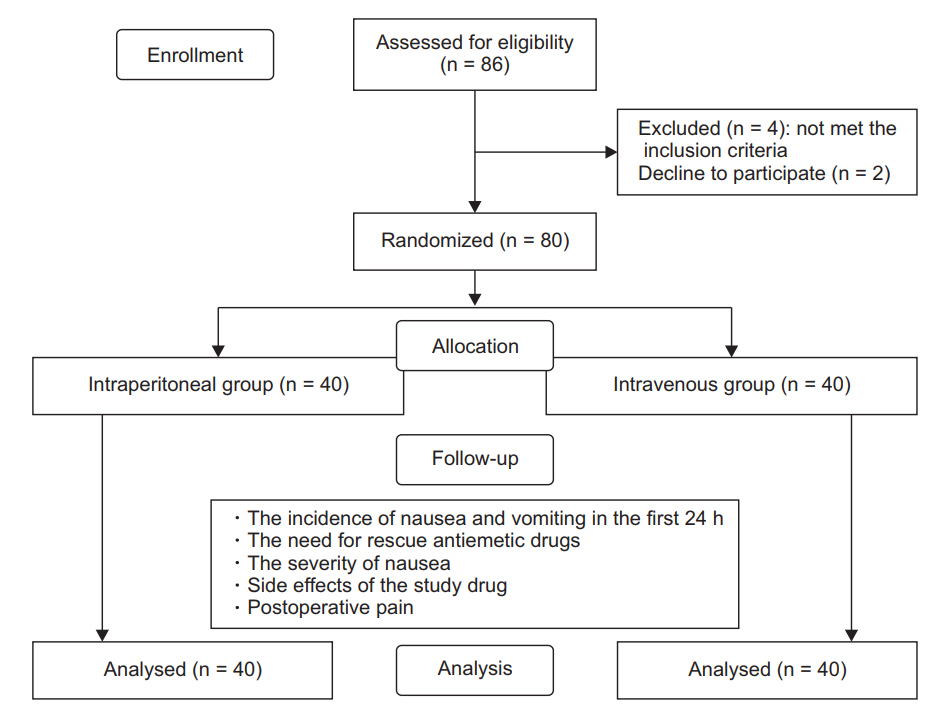
Eighty-six women were approached to participate in the study. Six women were excluded: 4 as they met various exclusion criteria, and 2 as they declined participation in the study. We randomly assigned the remaining 80 women into 2 groups (Fig. 1).
There were no significant differences in age, weight, height, Apfel risk score, duration of surgery, or duration of anesthesia between the 2 study groups (Table 1). The incidence of nausea in the IV group was 27.5% (11 patients) and that in the IP group was 7.5% (3 patients) in the first 24 h post-operatively (P = 0.037). There were no significant differences in the incidence of retching or vomiting, or the need for rescue antiemetics between the 2 groups (Table 2).
In terms of side effects, women in the IP group had a lower rate of side effects than those in the IV group (7.5% vs. 27.5%, P = 0.037). The most common side effects in the IV group were headache and dizziness (Table 3).
The mean VAS pain score and total meperidine consumption for the first 24 h postoperatively were lower in the IP group than in the IV group. However, these differences did not reach statistical significance (Table 4).
The current study showed that intraperitoneal dexamethasone significantly reduces the incidence of postoperative nausea, as compared to intravenous dexamethasone.
The mechanism by which dexamethasone protects against postoperative nausea remains unclear. The drug exerts its effect via the glucocorticoid receptor, which is present on many cells in the body [16]. Some glucocorticoid receptors are involved in the physiological transmission pathway for vomiting [17].
Other possible explanations for the prevention of postoperative nausea by dexamethasone includes central inhibition of prostaglandin synthesis, reduction of central serotonin activity, and change in the permeability of the blood–brain barrier to plasma proteins [18].
Although the mechanism underlying PONV is complex, it is mainly controlled by the vomiting center, which is the chemoreceptor trigger zone in the medulla that contains different receptors, such as those for serotonin 5-hydroxytryptamine type 3 (5-HT3), neurokinin 1, and dopamine [19].
PONV is a common complication affecting surgical patients [20]. Untreated PONV can increase the risk of some post-operative complications, such as gastric aspiration, bleeding, wound dehiscence, dehydration, and electrolyte disturbances [21]. Moreover, the duration of PACU stay could be prolonged, which would significantly increase the overall health care costs [2]. Therefore, PONV prophylaxis is necessary, especially in patients with elevated risk, such as females, patients on post-operative opioids, and those with a history of PONV [22].
Dexamethasone is effective in reducing PONV when used alone or in combination with other antiemetics. It is one of the most potent steroids available, with a half-life of 36–72 h [23]. Dexamethasone is an ideal drug, as it has a longer duration of action, is cheap and readily available, and is associated with earlier hospital discharge than other antiemetics [23,24]. Moreover, it could reduce postoperative pain by modulating anti-inflammatory mediators [25]. The main drawback of steroids is the possibility of serious adverse effects occurring from even a single dose [15]. Therefore, the use of a different route of administration was tested in the current study.
This study demonstrated that administration of 8 mg dexamethasone IP, at the end of gynecological laparoscopic procedures, significantly reduced postoperative nausea in the first 24 h, as compared to the same dose of IV dexamethasone. The difference in the incidence of postoperative nausea between the 2 groups in our study was directly related to the method of administration of dexamethasone, as the type of surgery, anesthesia protocol, the timing of administration, and the dose used were similar in both groups. We believe this to be a novel modality for reduction of postoperative nausea, as no similar studies have previously been reported.
Asgari et al. [13] reported that a single IP injection of 16 mg dexamethasone was associated with significant reduction in the severity of pain after gynecologic laparoscopy. Moreover, no complications, such as wound infection and delay in wound healing, were found among their participants. Similarly, in our study, no complications were present among the IP dexamethasone group. All the reported adverse effects were minor and were most probably related to recovery from general anesthesia.
The study had some limitations. Firstly, the small sample size could limit the generalizability of the results. Additionally, patients were lost to follow-up after 1 week, and thus we could not evaluate the occurrence of long-term side effects of dexamethasone.
Thus, we conclude that intraperitoneal dexamethasone at a dose of 8 mg at the end of gynecological laparoscopy reduces the incidence of postoperative nausea. Further studies are needed to compare the different timings and doses of dexamethasone administration in gynecological laparoscopy.
Table 1.
Patients' Clinical Characteristics
Table 2.
Incidence of Postoperative Nausea, Vomiting, and Retching, and Use of Rescue Antiemetics
Table 3.
Perioperative Adverse Events
Table 4.
Overall 24 hours VAS Score and Mean Meperidine Requirement during the First 24 hours Postoperatively
| Variable | IV group (n = 40) | IP group (n = 40) | P value |
|---|---|---|---|
| Overall 24 h VAS score | 3.1 ± 1.2 | 2.7 ± 1.1 | 0.210 |
| Mean meperidine requirement during 24 h (mg) | 86.4 ± 27.8 | 80.6 ± 18.9 | 0.278 |
References
1. Gan TJ, Diemunsch P, Habib AS, Kovac A, Kranke P, Meyer TA, et al. Consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg 2014; 118: 85-113.


2. Apfel CC, Korttila K, Abdalla M, Kerger H, Turan A, Vedder I, et al. A factorial trial of six interventions for the prevention of postoperative nausea and vomiting. N Engl J Med 2004; 350: 2441-51.



3. Habib AS, Gan TJ. Evidence-based management of postoperative nausea and vomiting: a review. Can J Anaesth 2004; 51: 326-41.


4. Apfel CC, Läärä E, Koivuranta M, Greim CA, Roewer N. A simplified risk score for predicting postoperative nausea and vomiting: conclusions from cross-validations between two centers. Anesthesiology 1999; 91: 693-700.


5. Sinclair DR, Chung F, Mezei G. Can postoperative nausea and vomiting be predicted? Anesthesiology 1999; 91: 109-18.


6. Karanicolas PJ, Smith SE, Kanbur B, Davies E, Guyatt GH. The impact of prophylactic dexamethasone on nausea and vomiting after laparoscopic cholecystectomy: a systematic review and meta-analysis. Ann Surg 2008; 248: 751-62.


7. Huang JC, Shieh JP, Tang CS, Tzeng JI, Chu KS, Wang JJ. Low-dose dexamethasone effectively prevents postoperative nausea and vomiting after ambulatory laparoscopic surgery. Can J Anaesth 2001; 48: 973-7.


8. Tzeng JI, Hsing CH, Chu CC, Chen YH, Wang JJ. Low-dose dexamethasone reduces nausea and vomiting after epidural morphine: a comparison of metoclopramide with saline. J Clin Anesth 2002; 14: 19-23.


9. Lee Y, Lin PC, Lai HY, Huang SJ, Lin YS, Cheng CR. Prevention of PONV with dexamethasone in female patients undergoing desflurane anesthesia for thyroidectomy. Acta Anaesthesiol Sin 2001; 39: 151-6.

10. De Oliveira GS Jr, Castro-Alves LJ, Ahmad S, Kendall MC, McCarthy RJ. Dexamethasone to prevent postoperative nausea and vomiting: an updated meta-analysis of randomized controlled trials. Anesth Analg 2013; 116: 58-74.


11. Bartlett R, Hartle AJ. Routine use of dexamethasone for postoperative nausea and vomiting: the case against. Anaesthesia 2013; 68: 892-6.


12. Assante J, Collins S, Hewer I. Infection associated with single-dose dexamethasone for prevention of postoperative nausea and vomiting: a literature review. AANA J 2015; 83: 281-8.

13. Asgari Z, Mozafar-Jalali S, Faridi-Tazehkand N, Sabet S. Intraperitoneal dexamethasone as a new method for relieving postoperative shoulder pain after gynecologic laparoscopy. Int J Fertil Steril 2012; 6: 59-64.


14. Asadollah S, Vahdat M, Yazdkhasti P, Nikravan N. The influence of dexamethasone on postoperative nausea and vomiting in patients undergoing gynecologic laparoscopic surgeries: A randomised, controlled, double blind trial. Turk J Obstet Gynecol 2014; 11: 219-23.




15. Bakri MH, Ismail EA, Ibrahim A. Comparison of dexmedetomidine and dexamethasone for prevention of postoperative nausea and vomiting after laparoscopic cholecystectomy. Korean J Anesthesiol 2015; 68: 254-60.



16. Holte K, Kehlet H. Perioperative single-dose glucocorticoid administration: pathophysiologic effects and clinical implications. J Am Coll Surg 2002; 195: 694-712.


17. Bountra C, Gale JD, Gardner CJ, Jordan CC, Kilpatrick GJ, Twissell DJ, et al. Towards understanding the aetiology and pathophysiology of the emetic reflex: novel approaches to antiemetic drugs. Oncology 1996; 53 Suppl 1: 102-9.


18. Henzi I, Walder B, Tramèr MR. Dexamethasone for the prevention of postoperative nausea and vomiting: a quantitative systematic review. Anesth Analg 2000; 90: 186-94.


19. Gan TJ. Mechanisms underlying postoperative nausea and vomiting and neurotransmitter receptor antagonist-based pharmacotherapy. CNS Drugs 2007; 21: 813-33.


20. Hamilton E, Ravikumar R, Bartlett D, Hepburn E, Hwang MJ, Mirza N, et al. Dexamethasone reduces emesis after major gastrointestinal surgery (DREAMS). Trials 2013; 14: 249.



21. Rose JB, Watcha MF. Postoperative nausea and vomiting in paediatric patients. Br J Anaesth 1999; 83: 104-17.



22. Si XY, Wu LP, Li XD, Li B, Zhou YM. Dexamethasone combined with other antiemetics for prophylaxis after laparoscopic cholecystectomy. Asian J Surg 2015; 38: 21-7.


23. Murphy GS, Szokol JW, Greenberg SB, Avram MJ, Vender JS, Nisman M, et al. Preoperative dexamethasone enhances quality of recovery after laparoscopic cholecystectomy: effect on in-hospital and postdischarge recovery outcomes. Anesthesiology 2011; 114: 882-90.