Cardiac arrest after sugammadex administration in a patient with variant angina: a case report
Article information
Abstract
A 76-year-old man with no notable medical history was scheduled for a robot-assisted radical prostatectomy. After the operation, he was given sugammadex. Two minutes later, ventricular premature contraction bigeminy began, followed by cardiac arrest. Cardiac arrest occurred three times and cardiopulmonary resuscitation was done. The patient recovered after the third cardiopulmonary resuscitation and was transferred to the intensive care unit. Coronary angiography was done on postoperative day 1. The patient was diagnosed with variant angina and discharged uneventfully on postoperative day 8.
Sugammadex is a modified gamma-cyclodextrin that is widely used as an antidote to rocuronium-induced neuromuscular blockade; it has been reported to be efficacious and safe [1]. A recent study, however, reported significant complications following sugammadex administration, such as hypersensitivity and anaphylaxis [234]. There has been only a single previous report on an occurrence of coronary spasm following sugammadex administration [5]. Here, we present a case of unexpected severe dysrhythmia and cardiac arrest after sugammadex administration in a patient with chest pain.
Case Report
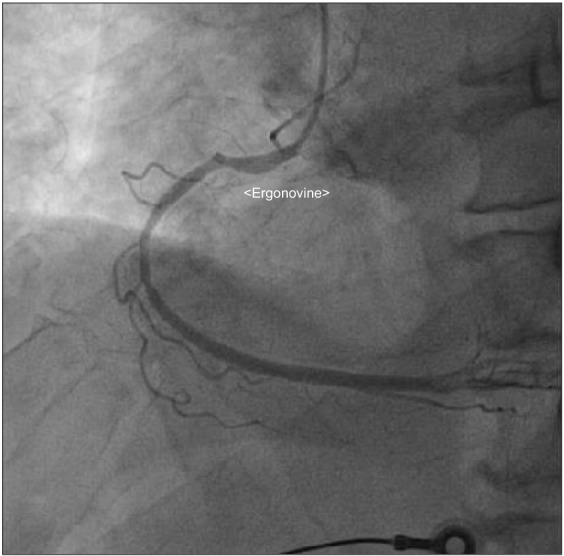
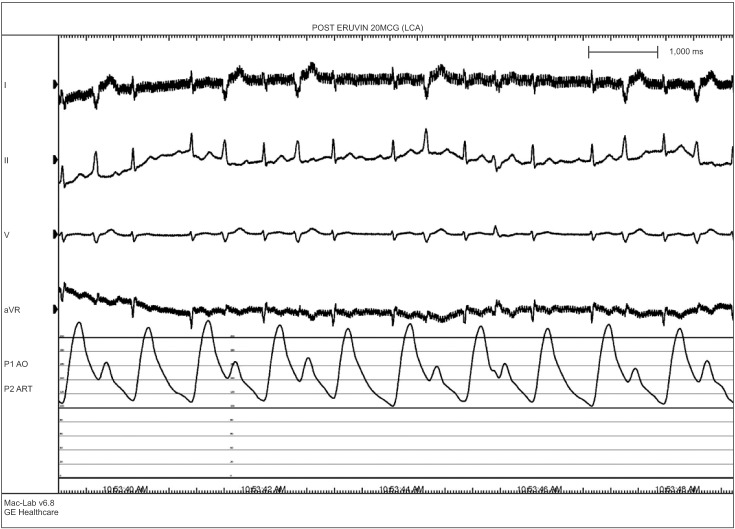
A 76-year-old male who was 169.7 cm tall and who weighed 65.2 kg was scheduled for robot-assisted radical prostatectomy under general anesthesia. He had no specific past medical history but suffered infrequent atypical chest pain. Preoperative transthoracic echocardiography (TTE) and an electrocardiogram (ECG) for cardiologic evaluation were done, with no abnormal findings except sinus bradycardia. The results of chest X-ray examination, pulmonary function testing, and other preoperative laboratory tests were normal. The patient entered the operating room without premedication. ECG, non-invasive blood pressure (NIBP), end-tidal carbon dioxide, and oxygen saturation (SpO2) were monitored. The patient's initial vital signs were: NIBP, 125/67 mmHg; SpO2, 98%; and heart rate, 64 beats/min. General anesthesia was induced with 120 mg propofol (Anepol®, Hana pharm, Hwaseong, Korea) and 50 mg rocuronium (Esmeron®, N.V. Organon, Oss, Netherlands). Mask ventilation was applied with 100% oxygen, and tracheal intubation was done without incident 2 minutes after rocuronium administration. The anesthesia was maintained with 6 volume % desflurane (Suprane®, Baxter Healthcare, Puerto Rico, USA) and remifentanil (Ultiva®, Glaxosmithkline, San Polo, Italy) infusion at 0.1 µg/kg/min. A right internal jugular vein catheter was inserted for fluid or blood administration, and the patient's continuous central venous pressure (CVP) was monitored. Invasive arterial blood pressure was monitored at the right radial artery, and the FloTrac/Vigileo™ system (Edwards Lifesciences, Irvine, CA, USA) was used to monitor the stroke volume variance (SVV) and cardiac index (CI). The operation was done in a steep Trendelenberg position at an angle of 45 degrees. The patient's intraoperative vital signs were maintained within the following ranges: systolic blood pressure, 90–100 mmHg; CVP, 10–15 mmHg; SVV, 10–15%; CI, 2.0–2.5; and body temperature, 35.5–36.0℃. The operation was completed uneventfully and took 4 hours and 15 minutes. At the time when the pneumoperitoneum was removed and the patient was taken out of the trendelenberg position, his blood pressure and heart rate had not changed significantly. An additional 50 mg of rocuronium was administered during the operation to maintain muscle relaxation, so the total dose of rocuronium was 100 mg. The total fluid input was 3,100 ml (500 ml colloid and 2,600 ml crystalloid), and the estimated bleeding count was 570 ml. We stopped administration of desflurane and remifentanil. After 5 minutes, the patient's train-of-four (TOF) was 2; we gave him 130 mg sugammadex (Bridion®, N.V. Organon, Oss, Netherlands). Two minutes later, sudden ventricular premature contraction (VPC) bigeminy appeared on the ECG, the heart rate decreased to below 40 /min, and the systolic blood pressure decreased to below 60 mmHg. Despite the administration of 10 mg ephedrine and 80 mg lidocaine i.v., the heart rate remained under 20 /min. After immediate chest compression for 10 seconds and administration of 0.5 mg atropine i.v., the patient's vital signs returned to the baseline values. Three µg/kg/min isosorbide dinitrate was infused to prevent myocardial ischemia. After 10 minutes, the patient's heart rate and blood pressure decreased, and we gave him 10 mg ephedrine. The patient did not respond, and cardiac arrest developed again. We initiated cardiopulmonary resuscitation (CPR) within seconds; other anesthesiology and cardiology staff arrived to help. During CPR, 1 mg epinephrine was given i.v., and ventricular tachycardia (VT) occurred. The patient was immediately cardioverted with 200 J, but he did not respond and CPR was continued. Over the 10 minutes during which CPR was performed, an additional dose of 1 mg epinephrine was given i.v. twice, cardioversion with 200 J was done twice, and 0.4 mg nitroglycerin was given i.v.; the patient's ECG showed a sinus rhythm of 110 /min. Dopamine and norepinephrine were infused to maintain normal blood pressure and cardiac output. After 10 minutes, VT recurred; 200 J cardioversion was delivered immediately and 2 g magnesium was given i.v. The patient recovered directly; his ECG showed a sinus rhythm with mild tachycardia and a heart rate of 90–100 /min. We monitored the patient for 30 minutes, during which time his vital signs were kept stable with dopamine infusion at 5 µg/kg/min and norepinephrine infusion at 0.25 µg/kg/min. The patient was transferred to the intensive care unit. His arterial blood gas and electrolytes were checked; all values were within the normal limits, including a serum magnesium level of 2.1 mg/dl. His myoglobin was elevated to 302.9 ng/ml, and his CK-MB/troponin I level was normal on the day of the operation. On postoperative day 1, his CK-MB/troponin I level was increased to 15.6/8.21 ng/ml; coronary angiography was done, which showed coronary artery obstructive disease. Concentric tubular stenotic lesions in the right coronary artery and left circumflex artery, which blocked 40% and 50% of the blood flow, respectively, were observed. Also, a coronary spasm was observed on the ergonovine test, and the patient was diagnosed with variant angina (Fig. 1). The ECG results of the VPC bigeminy obtained from the ergonovine test were similar to those obtained in the two minutes after sugammadex administration (Fig. 2). On postoperative day 1, the patient's trachea was extubated; oxygen saturation was maintained well with spontaneous respiration. On postoperative day 2, the patient's blood pressure was normal without vasopressors. The patient was transferred to the general ward on postoperative day 2. On postoperative day 8, the patient was discharged uneventfully with normal ECG and laboratory results. After 4 weeks, the patient visited our outpatient clinic; intradermal testing was done with a 1 : 100 dilution of sugammadex 100 mg/ml, rocuronium (1 : 50) and sugammadex-rocuronium complex, and the results were negative.
Discussion
In this case, the patient had three occurrences of sudden cardiac arrest after administration of sugammadex. CPR was done for about 40 minutes total; after the third CPR, the patient recovered and showed hemodynamic stability. The cause of cardiac arrest is unclear. However, coronary angiography with an ergonovine test showed variant angina postoperatively.
First, we presumed anaphylaxis to be the cause of cardiac arrest. Several cases of anaphylaxis or severe cardiac collapse after administration of sugammadex have been previously reported [234]. However, intradermal testing with sugammadex, rocuronium, and sugammadex-rocuronium complex showed negative results. In addition, the patient had no symptoms of an allergic reaction, such as a skin rash or angioedema. Therefore, the possibility of anaphylaxis was low.
Second, we considered that the cardiac arrest resulted from acute myocardial infarction accompanied by a coronary spasm. The patient had no specific medical history or smoking history. The preoperative laboratory results were also normal. However, the patient had infrequent chest pain preoperatively and was diagnosed with variant angina postoperatively. Factors that contribute to the development of coronary spasms are smoking, cocaine use, hypomagnesemia, insulin resistance, vitamin E deficiency, hyperventilation, cold stimulation, and administration of sumatriptan and ergotamine [6]. We suspected that the coronary spasm was caused by either hypomagnesemia or the administration of sugammadex. We were not able to measure the serum magnesium levels before and during the surgery. Thus, we could not determine whether hypomagnesemia developed. However, it is reasonable to assume that it did develop, because the patient's ECG and blood pressure stabilized after the administration of 2 g magnesium during the third cardiac arrest. The magnesium level measured after the surgery was 2.1 mg/dl, a low normal value, which indicates that the level was lower than the normal values before the administration of 2 g magnesium. However, it was not clear whether hypomagnesemia was a direct cause of the coronary spasm or an aggravating factor that indirectly sustained the arrhythmia.
We identified the administration of sugammadex as a more probable cause of the coronary spasm. VPC bigeminy appeared 2 minutes after sugammadex administration and severe hypotension; bradycardia occurred simultaneously. In addition, the ECG results of VPC bigeminy obtained 2 minutes after the administration of sugammadex were similar to those obtained from the ergonovine test. Therefore, we considered that the patient's severe dysrhythmia and cardiac arrest could have been triggered by sugammadex given the time course of drug administration. However, this is not absolute proof. There are few lines of evidence that suggest that sugammdex is associated with coronary spasms. As a modified gamma-cyclodextrin, sugammadex can reverse muscle relaxation by dissolving steroidal non-depolarizing neuromuscular blockades, especially those caused by rocuronium. The effects of sugammadex on the human cardiovascular system have rarely been studied. However, one study found that sugammadex has no effect on the release and metabolism of acetylcholine, unlike acetylcholinesterase inhibitors. Therefore, the hemodynamic effect of sugammadex should be minimal [1]. Another study found that sugammadex does not cause QT prolongation, which can lead to severe arrhythmia [7]. However, Hoshino et al. [5] reported a case of repetitive cardiac arrest due to coronary vasospasm after sugammadex administration. Also, there was one case in which coronary spasm was caused by administration of neostigmine, which was used to reverse muscle relaxation before sugammadex was developed [8]. However, the coronary spasm in that case was caused by an increased level of acetylcholine, and the mechanism of action of neostigmine is different from that of sugammadex. Our study has the limitation that we couldn't rule out hypomagnesemia as a cause of coronary spasm. Therefore, more studies and case reports are needed to determine how sugammadex can cause coronary spasm.
In conclusion, sugammadex may trigger coronary spasms in patients with variant angina. Thus, anesthesiologists should be cautious about administering sugammadex to such patients.