|
 |
|
|
Abstract
Massive hemoptysis is respiratory compromise which should be managed as a life-threatening condition. In our case, the bronchial blocker played a role in hemostasis of tracheal bleeding very close to the carina and prevented further spillage into the contralateral lung. Right-sided one-lung isolation in an 87-year-old female, who received cardiopulmonary resuscitation due to myocardial infarction, was requested due to hemoptysis. Right-sided bronchial bleeding was suspected on auscultation, but esophageal and tracheal bleeding due to violent intubation with a stylet was also considered. We attempted one-lung isolation with the bronchial blocker. The bronchial blocker was inadvertently advanced to the left mainstem bronchus, but the inflated balloon of the bronchial blocker compressed the site of bleeding, which was within 1 cm proximal and left posterior to the carina. Tracheal bleeding stopped, and we confirmed that hemostasis was achieved with the balloon of the bronchial blocker using a fiberoptic bronchoscope.
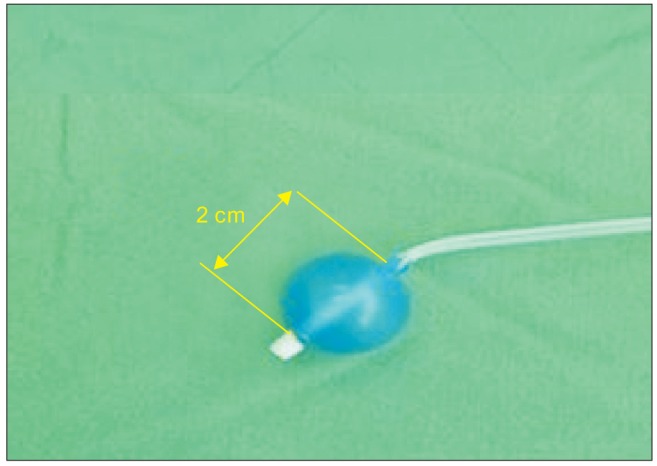
Hemoptysis is respiratory compromise with a wide clinical ranges from blood-tinged sputum to massive bleeding. Although its incidence is low, major hemoptysis should be managed as a life-threatening condition [1]. Therefore, prompt and appropriate management is required. Airway protection and volume resuscitation are critical steps for saving a life [1], but therapeutic measures including one-lung isolation (OLI) in an airway with profuse bleeding are not feasible to perform. The authors report hemostasis of tracheal bleeding very close to the carina using the bronchial blocker (Coopdech endobronchial blocker, Smiths-Medical, Barcelona, Spain) (BB) (Fig. 1) in a patient who underwent cardiopulmonary resuscitation (CPR) due to myocardial infarction.
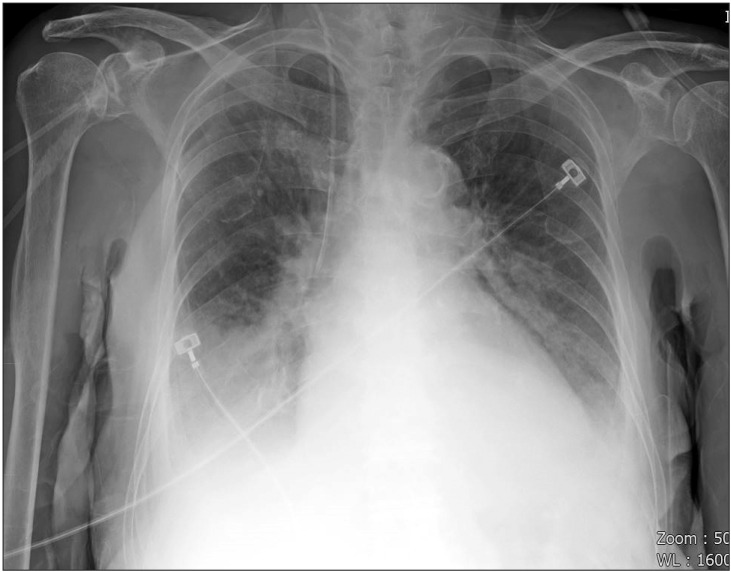
An 87-year-old female with a short neck (height 155 cm, weight 54 kg) was admitted to the hospital via the emergency room. She was taking medications for diabetes mellitus and hypertension. Transthoracic echocardiography showed ejection fraction 31.7%, aortic stenosis grade 4, severe diffuse hypokinesia, systolic dysfunction of the left ventricle, and bilateral pleural effusion. Chest X-ray showed bilateral pulmonary edema (Fig. 2) and the hemoglobin level was 10.4 g/dl. Upon admission to the emergency room, intubation with a 7.0 mm plain endotracheal tube (ETT) was performed by a physician. Coronary angiography with a left mainstem coronary artery stent was performed and 2 sessions of CPR were performed during coronary angiography. After recovery from CPR, she was admitted to the intensive care unit (ICU). Heparinization and administration of nitroglycerin, norepinephrine, dopamine, and dobutamine were initiated. Blood from the ETT was observed during suction. On the 2nd day of the ICU stay, the ETT was extubuted.
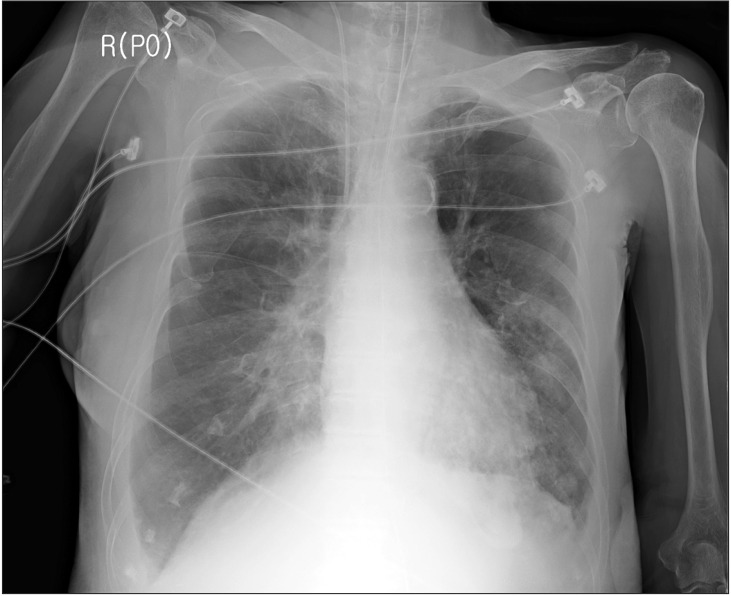
On the 5th day of the ICU stay, immediately after the patient returned from ultrasound-guided right pleural aspiration, blood gushed out from the oral cavity and O2 saturation dropped to 27%. Hypotension and bradycardia developed. Cardiac arrest occurred soon and CPR was initiated. At that time, a physician tried to perform endotracheal intubation with a stylet but she succeeded in performing intubation after several failures including esophageal intubation. After the first CPR, massive bleeding through the ETT and from the esophagus was observed and transfusion of blood products including packed RBCs was initiated during CPR. The second CPR was performed after some time. Bleeding in the right lung was suspected on auscultation (Fig. 3) and the hemoglobin level was 6.4 g/dl After recovery from the second CPR. Flexible bronchoscopy (FOB) was performed by a pulmonologist. He identified bleeding at the site just proximal and left posterior to the carina, which was presumed to be from an about 18 cm depth at the oral angle. However, the visual field of FOB was very poor due to profuse bleeding and advancement of the FOB into the distal tracheobronchial tree could not be executed. On emergent esophagogastroduodenoscopy, laceration was observed on the anterior and lower portions of the esophagus and bleeding from the laceration was also observed. After recovery from 2 sessions of CPR, myoclonus was observed and a neurologist suspected it as sequelae of hypoxic brain damage. Physicians considered that the main cause of persistent airway bleeding was bleeding in the right lung rather than the trachea and they requested OLI to the anesthesiologist.
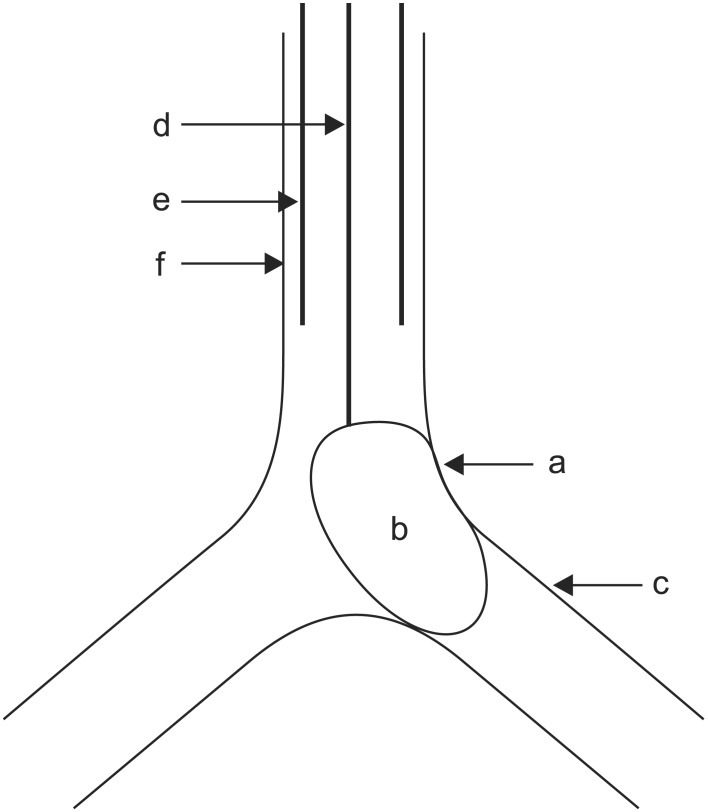
When anesthesiologists arrived in the ICU, CPR was in progress for 60 minutes. At 30 minutes after arrival of anesthesiologists, vital signs were as follows: blood pressure (BP) 90/65 mmHg, heart rate (HR) 73 beats/min, and O2 saturation 67%, and then the anesthesiologists attempted OLI. Considering persistent bleeding and suspected airway injury caused by violent and failed intubation, there were concerns about additional airway injury when the preexisting single lumen ETT was replaced with larger diameter ETT or the double lumen tube (DLT). In addition, because too much blood gushed out from the esophagus, it was impossible to extubate the preexisting single lumen ETT due to concerns about additional asphyxia. Therefore, we decided to place the BB through the preexisting single lumen ETT. At first, we inserted the BB under the guidance of an FOB of 3.1 mm diameter but the visual field was not properly secured due to massive bleeding. In addition, 7.5 mm diameter of the ETT was not large enough to simultaneously insert the BB and the FOB, due to sticky blood clot within the lumen of the ETT. Because we could not guide the tip of the BB under FOB, we had to blindly guide the tip of the BB into position according to the results of FOB by the pulmonologist. However, the left lung was blocked on auscultation, contrary to our intention when the balloon of the BB was inflated with 8 ml air. Therefore, we inserted the FOB to reposition the tip of the BB after suctioning the blood in the airway. At that time, the balloon of the BB compressed the site of bleeding, which was within 1 cm proximal and left posterior to the carina (Fig. 4). Bleeding was dramatically decreased and the visual field was much improved than that before the insertion of the BB. The amount of bleeding through the ETT that came out by suction was greatly decreased. Ten minutes after placement of the BB, vital signs were as follows: BP 125/84 mmHg, HR 97 beats/min, and O2 saturation 97%. We tried to suction the blood through the tip of the BB for confirmation of bleeding in the left lung but blood was not suctioned.
To control the bleeding in the trachea, we requested consultation with thoracic surgeons but they refused to perform surgery because of unstable patient status including recent recovery after myocardial infarction, heparinization, and post-CPR state. Finally, we decided to observe the patient under Do Not Resuscitate orders and administered midazolam 5 mg for sedation. Anesthesiologists confirmed stable vital signs for 20 minutes and came out from the ICU. The patient maintained stable vital signs for about 1 hours with the use of an Ambu bag after placement of the BB. Then the patient was shifted to mechanical ventilation (pressure controlled ventilation) with administration of cisatracurium 10 mg, and she showed an O2 saturation of 99%. Ten units of packed RBCs, 4 units of fresh frozen plasma, and 10 units of platelet concentrates were transfused from the initial CPR on the 5th day of the ICU stay. However, immediately after conversion to mechanical ventilation, the patient showed seizure-like motion on light touch. About 15 minutes after conversion, systolic BP was suddenly decreased to 70 mmHg and O2 saturation was decreased to 70%. The vital signs were not improved regardless of the use of vasopressors and 100% O2. Massive bleeding through the ETT was observed again, and airway pressure was suddenly increased and tidal volume was decreased. HR decreased to less than 40 beats/min and cardiac arrest occurred again. Finally, the patient expired 120 min after placement of the BB.
Massive hemoptysis should be managed with airway protection and hemodynamic stabilization [1]. For airway protection in hemoptysis, OLI is required, but difficulties might exist according to several clinical circumstances including poor visual field due to bleeding, the size of preexisting single lumen ETT, difficult airway, small airway, physician's inexperience with airway devices, unstable vital status, and possibility of airway injury [1,2,3]. In our case, cardiac arrest occurred and hemoptysis was managed with violent and urgent intubation using a 7.5 mm single lumen ETT. Because tracheal and esophageal injury caused by violent and inexperienced intubation was suspected, we had to exclude switching of the preexisting single lumen ETT to a larger diameter ETT or the DLT [1,4] and we selected the BB for OLI. However, it was also difficult to place the BB under FOB because of the poor visual field caused by profuse bleeding from the airway and esophagus. Although there is no clear cutoff value of massive hemoptysis, massive hemoptysis should always be considered as a lethal condition [1,5]. As shown in this case, massive bleeding from the airway and esophagus led to unstable vital signs and large amount of blood products had to be transfused due to unstable hemodynamics. Massive bleeding might contribute to consequent cardiac arrest in the patient with serious underlying conditions including severe aortic stenosis and recent myocardial infarction.
Chest X-ray, computed tomography (CT), digital subtraction angiography, and flexible/rigid bronchoscopy are necessary for diagnosis of hemoptysis [1,6]. When it is not possible to identify a localized finding in the chest X-ray such as in our case (Figs. 2 and 3), other diagnostic tools including bronchoscopy and CT are necessary [6]. CT is a highly useful tool in evaluation of hemoptysis, but it has limitations in patients with unstable hemodynamics, active bleeding requiring endobronchial management, and bilateral lung abnormalities [1,6]. The patient could not be shifted to the CT room because of unstable hemodynamics, resuscitation, and active bleeding [1]. Digital subtraction angiography has an important role in diagnosis of hemoptysis. It is performed when endovascular treatment is scheduled and other diagnostic modalities have been used [6]. Rigid bronchoscopy is more efficient than FOB in many aspects, including better clearing of the airway, better visualization, protecting airway patency, and maintaining ventilation [7]. However, we performed flexible bronchoscopy due to preexisting ETT and suspected airway injury. In addition, FOB can confirm the site of bleeding only in 73%ŌĆō93% episodes of massive bleeding [8,9] and definitive diagnosis of the bleeding site in our case could not be established before placement of the BB because of massive bleeding.
The BB has advantages in patients with a difficult airway, tracheostomy, unplanned OLI, and mechanical ventilation after surgery [10,11,12]. In our case, preexisting ETT, ongoing airway bleeding, and suspected airway injury led to the selection of the BB for OLI instead of the DLT. When the DLT cannot be an option for OLI, the BB can be an alternative [2]. However, malposition of the BB occurs more often than that of the DLT [13]. Malpositioned balloon of the BB might cause spillage of blood into both lungs and consequent hypoxemia and the underlying conditions might be aggravated. Malposition of the BB can cause serious events, such as ventilation problems or cardiac arrest [14]. In addition, placement of the BB may require longer time than placement of the DLT [15]. Therefore, in the emergent situation such as massive hemoptysis, securing the airway using the BB can be time-consuming and it can impede the treatment, particularly by inexperienced physicians. In our case, malposition of the BB was suspected due to seizure-like motion on light touch, unidentified patient movement under light sedation, or movement of the BB by medical personnel during switching to mechanical ventilation. However, physicians who were not familiar with the BB might not have noticed the exact cause of the ventilation problem. When blood came out through the ETT again, the possibility of malposition of the BB should have been considered. Medical personnel in the ICU did not check for proper positioning of the BB at regular intervals. It was thought that the patient expired due to ventilation failure and hypoxia caused by suffocation, and thereby aggravation of the underlying conditions.
In our case, balloon of the BB inadvertently compressed the site of tracheal bleeding and the degree of bleeding through the ETT was greatly decreased (Figs. 1 and 4). Therefore, we could save considerable time (about 1 hours) for additional steps of treatment. Various therapeutic measures exist as follows: cold saline lavage, topical vasoconstrictive agents, tranexamic acid, fibrinogen/fibrin, balloon tamponade, laser photocoagulation, electrocautery, bronchial artery embolization, and surgery [1]. However, serious medical conditions and unavailability of equipments/agents have limited the therapeutic options. Especially, myocardial infarction, heparinization, and post-CPR state made us hesitant.
In conclusion, tracheal bleeding very close to the carina might be temporarily controlled using the BB such as in our case. However, considering the possibility of malposition of the BB, frequent confirmation of the BB position and more definite treatment should be pursued during temporary hemostasis.
References
1. Sakr L, Dutau H. Massive hemoptysis: an update on the role of bronchoscopy in diagnosis and management. Respiration 2010; 80: 38-58. PMID: 20090288.


2. Brodsky JB. Lung separation and the difficult airway. Br J Anaesth 2009; 103(Suppl 1): i66-i75. PMID: 20007992.



3. Dumans-Nizard V, Liu N, Laloë PA, Fischler M. A comparison of the deflecting-tip bronchial blocker with a wire-guided blocker or left-sided double-lumen tube. J Cardiothorac Vasc Anesth 2009; 23: 501-505. PMID: 19362014.


4. Slinger RD, Campos JH. Anesthesia for thoracic surgery. Edited by Miller RDMiller's Anesthesia. 7th ed. : Philadelphia, Elsevier Churchill Livingstone. 2010, pp 1819-1887.
5. Dweik RA, Stoller JK. Role of bronchoscopy in massive hemoptysis. Clin Chest Med 1999; 20: 89-105. PMID: 10205720.


6. Larici AR, Franchi P, Occhipinti M, Contegiacomo A, del Ciello A, Calandriello L, et al. Diagnosis and management of hemoptysis. Diagn Interv Radiol 2014; 20: 299-309. PMID: 24808437.



7. Conlan AA, Hurwitz SS. Management of massive haemoptysis with the rigid bronchoscope and cold saline lavage. Thorax 1980; 35: 901-904. PMID: 7268664.



8. Revel MP, Fournier LS, Hennebicque AS, Cuenod CA, Meyer G, Reynaud P, et al. Can CT replace bronchoscopy in the detection of the site and cause of bleeding in patients with large or massive hemoptysis? AJR Am J Roentgenol 2002; 179: 1217-1224. PMID: 12388502.


9. Hsiao EI, Kirsch CM, Kagawa FT, Wehner JH, Jensen WA, Baxter RB. Utility of fiberoptic bronchoscopy before bronchial artery embolization for massive hemoptysis. AJR Am J Roentgenol 2001; 177: 861-867. PMID: 11566690.


10. Campos JH. Lung isolation techniques for patients with difficult airway. Curr Opin Anaesthesiol 2010; 23: 12-17. PMID: 19752725.


11. Benumof JL. Difficult tubes and difficult airways. J Cardiothorac Vasc Anesth 1998; 12(2): 131-132. PMID: 9583539.


12. Ho┼¤ten T, G├╝rkan Y, Sahillio─¤lu E, Top├¦u S, Solak M, Toker K. Our bronchial blocker experiences in one lung ventilation. Tuberk Toraks 2009; 57: 155-162. PMID: 19714506.

13. Narayanaswamy M, McRae K, Slinger P, Dugas G, Kanellakos GW, Roscoe A, et al. Choosing a lung isolation device for thoracic surgery: a randomized trial of three bronchial blockers versus double-lumen tubes. Anesth Analg 2009; 108: 1097-1101. PMID: 19299767.


14. Sandberg WS. Endobronchial blocker dislodgement leading to pulseless electrical activity. Anesth Analg 2005; 100: 1728-1730. PMID: 15920204.


15. Campos JH, Hallam EA, Van Natta T, Kernstine KH. Devices for lung isolation used by anesthesiologists with limited thoracic experience: comparison of double-lumen endotracheal tube, Univent torque control blocker, and Arndt wire-guided endobronchial blocker. Anesthesiology 2006; 104: 261-266. PMID: 16436844.


- TOOLS
-
METRICS

-
- 0 Crossref
- 1 Scopus
- 4,599 View
- 39 Download