A comparison of i-gel™ and LMA Supreme™ in anesthetized and paralyzed children
Article information
Abstract
Background
Both the i-gel™ (i-gel) and LMA Supreme™ (Supreme) are new single-use second generation supraglottic airway devices available in pediatric sizes. This study was designed to investigate the i-gel in comparison with the Supreme in children undergoing general anesthesia.
Methods
One hundred children with American Society of Anesthesiologists physical status I or II undergoing general anesthesia were randomly assigned to either the i-gel or the Supreme group (50 children in each group). The device size was chosen according to weight of the children. We assessed the insertion success rate, insertion time, oropharyngeal leak pressure, grade of the fiberoptic glottic view, number of airway manipulations required, and postoperative complications.
Results
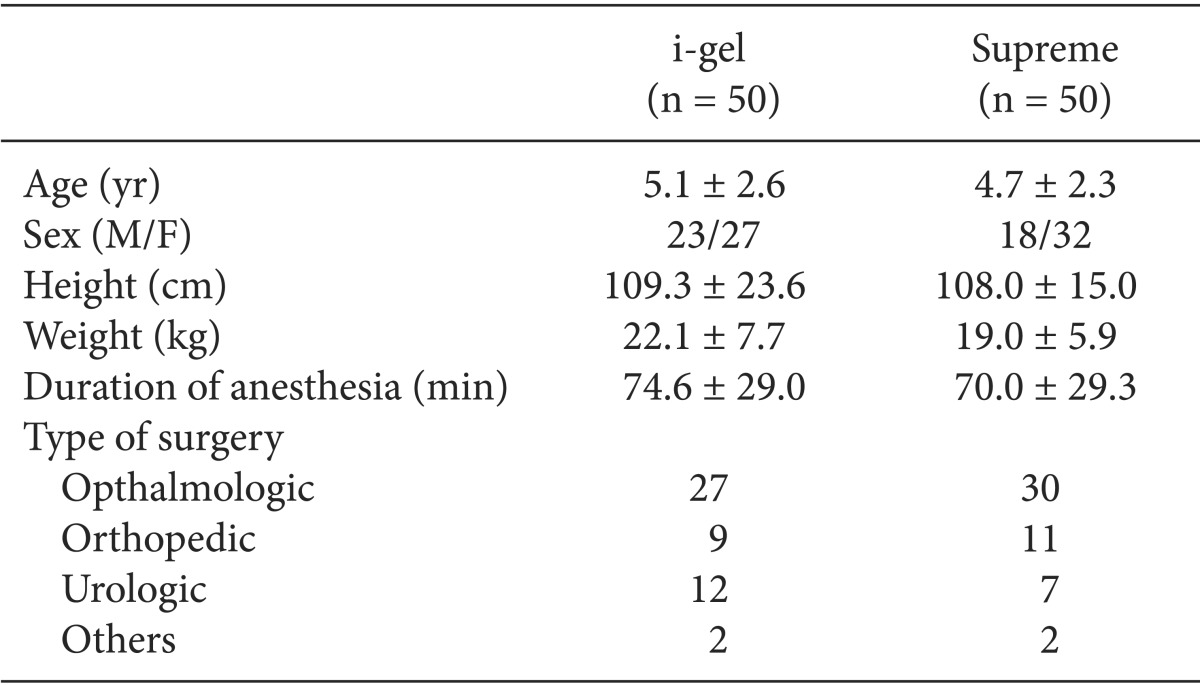
There were no differences in the demographic data between the two groups. The success rate of insertion was same in both groups. The insertion time of the i-gel was longer than that of Supreme (P = 0.004). The oropharyngeal leak pressure in the i-gel group was higher than that in the Supreme group (P = 0.013). On fiberoptic examination, the vocal cords were visible in 90% of the children in the i-gel group and in 96% of the children in the Supreme group. The number of airway manipulations required was higher in the i-gel group (14 cases) than in the Supreme group (1 case) (P < 0.001). There were no differences in complications including blood staining of the device and sore throat between both groups.
Conclusions
Both the i-gel and Supreme provided a satisfactory airway during general anesthesia in children. Compared to the Supreme, the i-gel demonstrated a higher oropharyngeal leak pressure, longer time for insertion, and a greater number of airway manipulations during anesthesia.
Introduction
Both the i-gel™ (i-gel) and LMA Supreme™ (Supreme) are new, single-use, second-generation, supraglottic airway devices (SAD) available in pediatric sizes. The i-gel (Intersurgical Ltd., Wokingham, Berkshire, UK) is a latex-free SAD with a non-inflatable cuff and a gastric drain tube. The Supreme (The Laryngeal Mask Company Ltd., St Helier, Jersey, UK) has a curved and rigid airway tube, a drain tube positioned within the center of the airway tube, and a relatively large inflatable cuff made of polyvinyl chloride.
There are many studies comparing the Supreme with other SADs that have shown effective clinical performances in adults and children [1,2,3,4,5,6,7]. The pediatric i-gel (sizes 1.0, 1.5, 2.0, and 2.5) has recently become available commercially and there are a few published studies to date [7,8,9,10,11]. These types of studies are particularly important in children because they are known to be more vulnerable to complications related to the use of SADs [12,13]. There are a few studies comparing between the i-gel and the Supreme in children.
This study was designed to investigate the i-gel in comparison with the Supreme, the latest and reliable LMA, in children undergoing general anesthesia. Our main aim was to compare the oropharyngeal leak pressure between the two groups. We also assessed the insertion success rate, insertion time, airway quality during maintenance of anesthesia, number of airway manipulations required for adequate ventilation, and postoperative complications.
Materials and Methods
The Institutional Review Board approval was obtained for this study, and in all cases, written informed consent was obtained from the parents. The children included in the study were aged 1-15 years, had American Society of Anesthesiologists physical status I or II, weight 5-30 kg, and scheduled for elective surgeries within 2 hours under general anesthesia. Children at risk of regurgitation or those with known airway abnormalities were excluded.
In this study, two anesthesiologists performed all insertions of the i-gel and Supreme. Prior to this study, they had placed the i-gel and Supreme in 10 children each, but they had significant experience in placing the i-gel and Supreme in many adults (over 100 patients).
One hundred children were randomized by a computergenerated list to receive either the i-gel or the Supreme, and to be assigned to the groups of two anesthesiologists (Fig. 1). The size of the airway device selected was based on the patient's body weight according to the manufacturer's recommendations (i-gel: size 1.5, 5-12 kg; size 2, 10-25 kg; size 2.5, 20-35 kg. Supreme: size 1.5, 5-10 kg; size 2, 10-20 kg; size 2.5, 20-30 kg).
Anesthesia was induced with an intravenous injection of ketamine 2 mg/kg and rocuronium 0.6 mg/kg. Once an adequate depth of anesthesia was achieved (no eyelash reflex and no response to jaw thrust) [14], the i-gel or the Supreme was inserted by the standard technique recommended by the manufacturer. The insertion time was measured from the moment the face mask was removed from the patient's face until the first capnography upstroke. The intracuff pressure of Supreme was then set to 60 cmH2O using a cuff pressure manometer (Mallinckrodt Medical, Athlone, Ireland). Correct insertion was assessed by proper chest expansion, the presence of CO2 wave form with a plateau on the capnograph, absence of audible leak, and lack of gastric insufflation determined by neck and epigastric auscultation.
We recorded the number of insertion attempts, the insertion time, the number and type of airway manipulations required to establish an adequate airway (such as pushing or pulling the device, chin lift, or jaw thrust), and complications induced by insertion.
Anesthesia was maintained with sevoflurane and N2O. Supplementary rocuronium was administered, if needed. Children's lungs were ventilated with a tidal volume of 10 ml/kg at a respiratory rate between 14 and 20 bpm. It was adjusted to maintain an end-tidal CO2 of 35-40 mmHg. The airway sealing was examined by measuring the oropharyngeal leak pressure using the manometer on the anesthetic breathing system (Primus, Drager, Lubeck, Germany) with the adjustable pressure-limiting valve set at 30 cmH2O and a oxygen fresh gas flow (FiO2 1.0) of 3 L/min and the point of gas leak determined by auscultation was recorded. If there was airway leakage, we inflated the cuff more before manipulation of the Supreme, while we manipulated the i-gel instantly.
The anatomical position of the airway was evaluated by assessing the fiberoptic glottic view through the airway. The fiberoptic glottic view was graded from 1 to 4 as follows: grade 1, not visible at all; grade 2, the only epiglottis visible; grade 3, arytenoid and partial part of vocal cords visible; grade 4, whole vocal cords visible [10].
During maintenance of anesthesia, we recorded oxygen saturation, end-tidal volume, peak inspiratory pressure, number of airway manipulations required, airway complications (such as pulse oxygen saturation below 92% or severe airway leakage with expiratory tidal volume below 5 ml/kg), and the airway quality by performing neck auscultation. Airway quality was classified as clear, minimal obstruction, partial obstruction, and complete obstruction [15].
At the end of the surgery, anesthetic agents were discontinued and the device was removed after the child was awake. In this phase, complications, such as coughing, laryngospasm, bronchospasm, desaturation (SpO2 < 92%), and blood stain on the device after removal, were also recorded. An interview of the parents and children, if possible, was conducted immediately after recovery and 24 hours later, to evaluate postoperative complications such as dysphagia, dysphonia, dyspnea, and sore throat.
Sample size was based on data from a pilot study of 15 patients, in whom the mean oropharyngeal leak pressure with the i-gel was measured (mean ± SD; 25.4 ± 3.5 cmH2O) and compared with preview data for the Supreme [16]. To detect a difference of 10% in the mean oropharyngeal leak pressure between the groups with type I error of 0.05 and a power of 90%, a sample size of 42 patients was necessary. The study enrolled 100 patients (50 in each group) to allow for potential dropout.
Data are presented as mean ± SD or the number of patients. Statistical comparisons between the devices were made using the Student's t-test for continuous data, and chi-square test or Fisher's exact test for categorical data. A P < 0.05 was considered statistically significant.
Results
One hundred patients were recruited in this study. The results of all patients were included in this study analysis. There were no significant differences in demographic data between the two groups (Table 1).
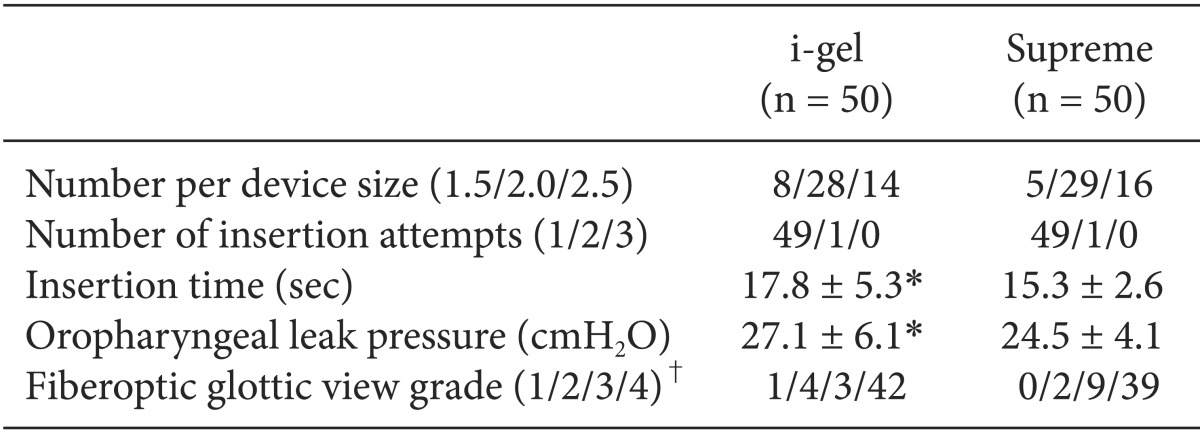
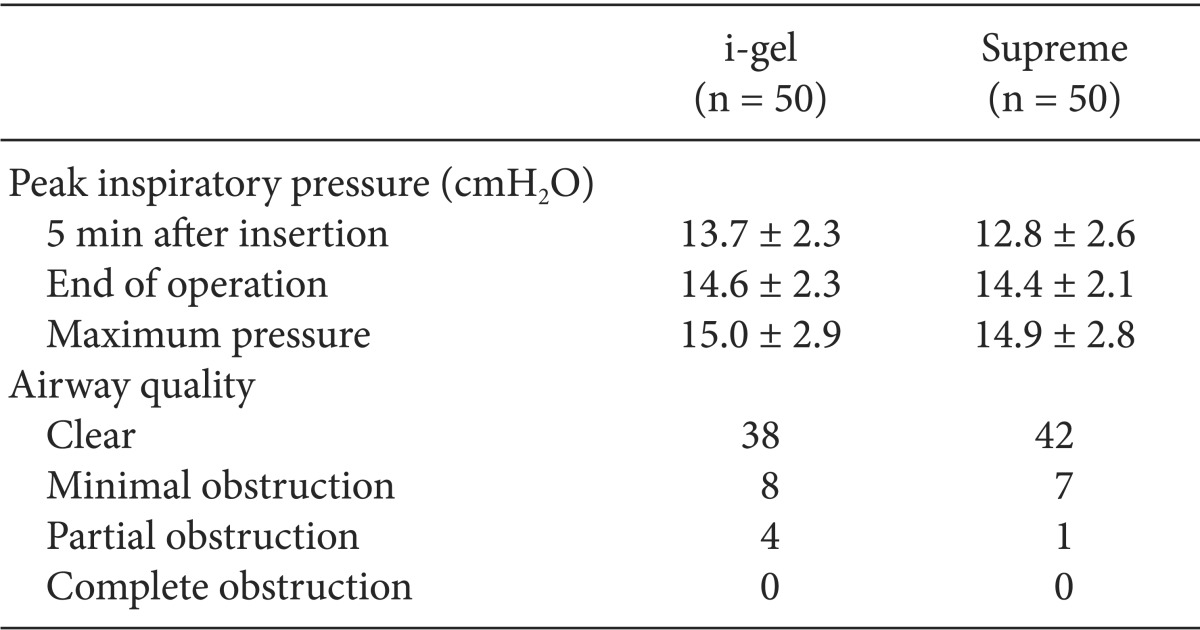
The comparative data for each device are described in Table 2. There were no statistically significant differences between the two groups in the number per device size, the insertion success rates, and the grade of fiberoptic glottic view. Almost all of the devices were inserted in the first attempt, and in only one patient in each group, a second attempt was needed for placement. There were no cases of failure and no cases requiring change of the device. The insertion time of the i-gel was longer than that of the Supreme (P = 0.004). Oropharyngeal leak pressures were higher with the i-gel than with the Supreme (P = 0.013). On fiberoptic examination, the vocal cords were visible in 90% of the children in the i-gel group and in 96% of the children in the Supreme group. The peak inspiratory pressure and airway quality were similar between the groups (Table 3).
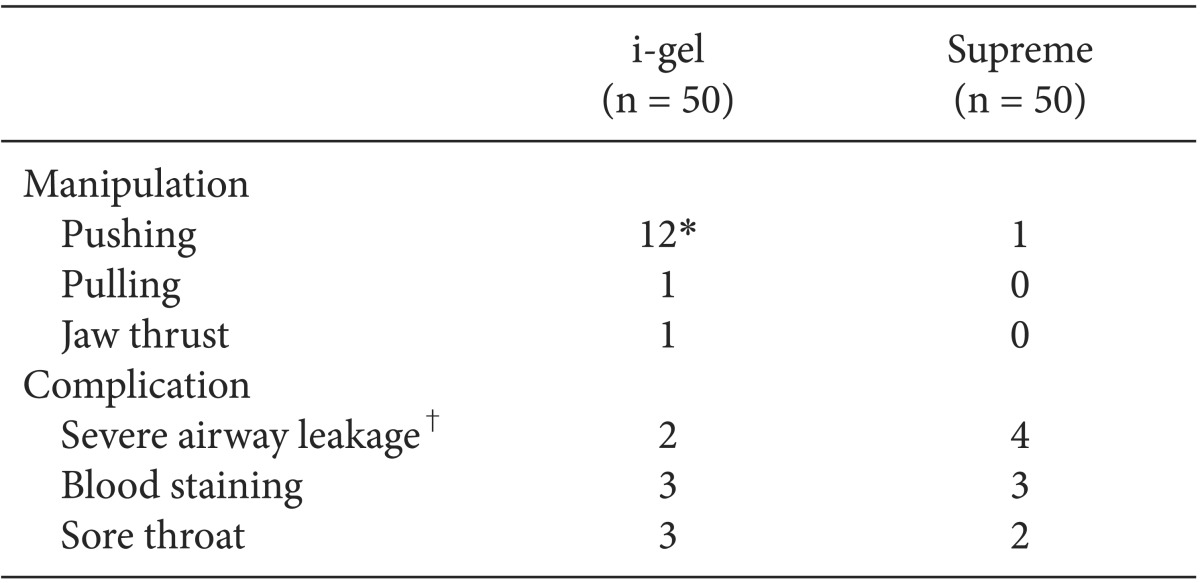
To resolve the airway leakage during maintenance, airway manipulations were required in 14 (28%) children of the i-gel group, but in only 1 (2%) child of the Supreme group (P < 0.001). Severe airway leakage occurred in 2 children of the i-gel group and in 4 children of the Supreme group. Three cases of severe airway leakage in the Supreme group were resolved with more inflation of the cuff, and 1 case of severe airway leakage in the Supreme group was resolved by pushing the device.
There were no significant postoperative complications in both groups. Blood staining on the device was observed in 3 (6%) children of the i-gel group and in 3 (6%) children of the Supreme group. Postoperative sore throat was noted in 3 (6%) children of the i-gel group and in 2 (4%) children of the Supreme group (Table 4).
Discussion
This study shows that the i-gel is a reliable airway device in children, similar to that in other studies in adults which have shown that the i-gel is associated with an easy insertion, high success rate and oropharyngeal leak pressure, and low rate of complications [17,18,19,20,21].
In this study, we found that insertion of the i-gel and Supreme was mostly successful on the first attempt except in one case in each group due to the patient's small mouth and large tongue, and all devices were successfully inserted within two attempts. These results are comparable with those in a study reported recently by Lee et al. [10], comparing the size 1.5-2.5 i-gel and the LMA Classic in children weighing between 5 and 30 kg. Goyal et al. [22] also reported that the success rate of the i-gel was excellent, which is similar to that in our study.
Shorter insertion times affect the feasibility of SADs. In a comparison of the i-gel with the Supreme in adults, the time taken to obtain adequate ventilation through the i-gel was longer than that through the Supreme [21,23] and these results are comparable with those in our study. Longer time of insertion for the i-gel may be due to the bulky shape of the device compared with the deflated cuff of the Supreme. Also, the Supreme has a 90 degree tube angle, designed to reduce stress on the upper jaw, in contrast to the slightly curved angle of the i-gel.
Oropharyngeal leak pressure is one of the important factors that determine the efficacy of a SAD [9]. Higher oropharyngeal leak pressure and lower peak inspiratory pressure may be advantageous in positive pressure ventilation, especially in obesity patients, lithotomy position, laparoscopic surgery, and restrictive pulmonary disease [24]. However, comparison of oropharyngeal leak pressures is complicated by differences in the method and presentation of results. We reported that oropharyngeal leak pressures were higher with the i-gel than with the Supreme, and this result was similar to that in the study by Jagannathan et al. [15]. But this difference in oropharyngeal leak pressure may not have clinical implications, and therefore, ventilation parameters were adequate in all of the patients.
Although different scoring systems were used for the laryngeal view in different studies, the cohort study by Beringer et al. as well as a number of adult studies [9,19,21] reported that the i-gel provided an improved fiberoptic glottic view compared with that provided by other SADs, and our study shows similar results to these studies. Although there is little or no correlation between device position as assessed fiberoptically [25,26], recently, SADs have been recommended as a conduit for tracheal tube insertion in cases of difficult intubation [27], and therefore, an improved glottic view is useful.
Airway manipulations to maintain adequate ventilation were needed much more frequently with the i-gel in this study. During maintenance of anesthesia, 28% of children in the i-gel group required advancement and re-taping of the device because of airway leakage or dislocation of the tube, compared to only 2% of children in the Supreme group. These findings were also seen in other studies with the pediatric i-gel [8,15,28]. We have realized that downward force with taping reduces this dislocation [28]. The sealing effect caused by this downward traction force is uncertain and could be a subject of further study.
The incidence of complications was low in all cases, except for blood staining in a few children of the i-gel and Supreme groups, but it is unlikely to be clinical important or statistically significant. Other studies have also reported a similar incidence of complications [7,9,10,11].
The longer insertion time and the higher number of manipulations of the i-gel may indicate difficult insertion compared to the Supreme. But once this device is placed correctly and fixed securely, the higher oropharyngeal leak pressure and low rate of complications suggest that the i-gel is good for maintaining positive pressure ventilation in children.
Our study has several limitations. The i-gel and Supreme have gastric channels, but we did not attempt to evaluate their usefulness. Also, all data were collected by unblinded investigators. We studied only healthy children with normal airways and lungs, and therefore, these results cannot be generalized to other vulnerable groups. Although there were no statistically significant differences between the findings recorded by the two anesthesiologists, they had different levels of experience and skills, and this may have introduced some bias in the results. Finally, some complications such as sore throat could not be evaluated in very young children.
In conclusion, both the i-gel and Supreme provided a satisfactory airway during general anesthesia in children. The i-gel demonstrated a higher oropharyngeal leak pressure, longer time for insertion, and a greater number of airway manipulations during anesthesia. Hence, the clinician should pay attention to these results when using this device for maintenance of anesthesia in children.