Risk score for postoperative complications in thoracic surgery
Article information
Abstract
Background
Risk scoring system for thoracic surgery patients have not been widely used, as of recently. We tried to forge a risk scoring system that predicts the risk of postoperative complications in patients undergoing major thoracic surgery. We used a prolonged ICU stay as a representative of postoperative complications and tested various possible risk factors for its relation.
Methods
Data from all patients who underwent major lung and esophageal cancer surgeries, between 2005 and 2007 in our hospital, were collected retrospectively (n = 858). Multiple logistic regression analysis was performed with various possible risk factors to build the risk scoring system for prolonged ICU stay (> 3 days).
Results
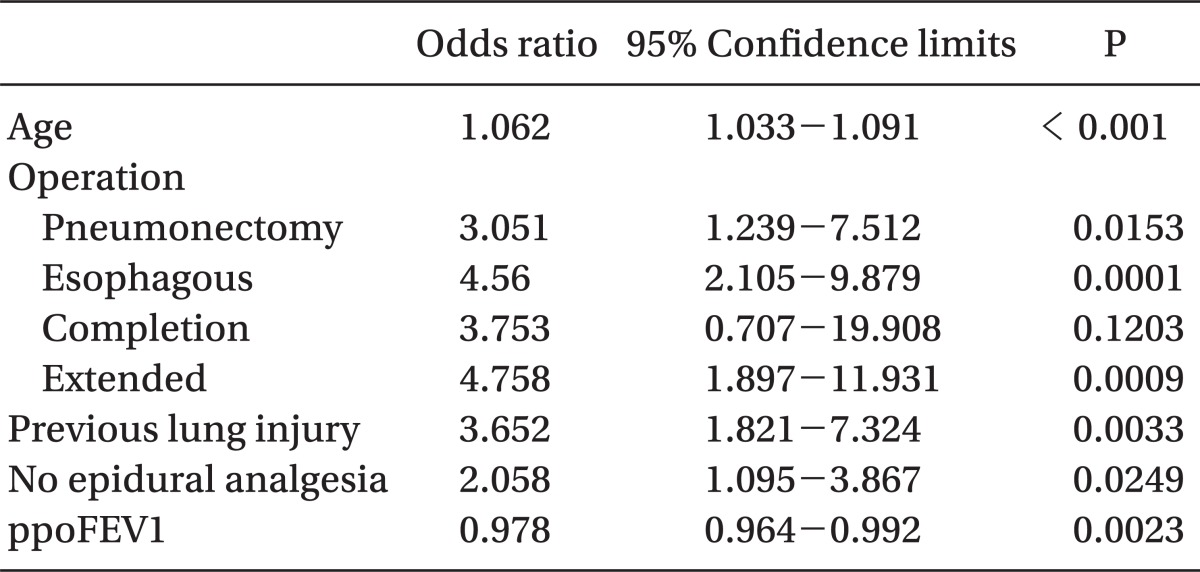
A total of 9% of patients exhibited more than 3 days of ICU stay. Age, operation name, preoperative lung injury, no epidural analgesia, and predicted post operative forced expiratory volume in 1 second (ppoFEV1) were the risk factors for prolonged ICU stay, by multivariable analysis (P < 0.05). Risk score, p was derived from the formula: logit(p/[1-p]) = -5.39 + 0.06 × age + 1.12 × operation name(2) + 1.52 × operation name(3) + 1.32 × operation name(4) + 1.56 × operation name(5) + 1.30 × preoperative lung injury + 0.72 × no epidural analgesia - 0.02 × ppoFEV1 [Age in years, operation name(2): pneumonectomy, operation name(3): esophageal cancer operation, operation name(4): completion pneumonectomy, operation name(5): extended operation, preoperative lung injury(+), epidural analgesia(-), ppoFEV1 in %].
Conclusions
Age, operation name, preoperative lung injury, epidural analgesia, and ppoFEV1 can predict postoperative morbidity in thoracic surgery patients.
Introduction
A large portion of patients undergoing thoracic surgery develops postoperative complications. Major respiratory complications occur in 20% and cardiac complications occur in 15% of the thoracic population [1]. If we know the risk of postoperative complications of a certain patient, based on an objective scoring system, we can obtain better patient's consent on their anesthetic and surgical risks, and focus our attention and resources on higher risk patients.
Cardiac surgeons have led the field of risk stratification [2], and Euro-SCORE system has been used widely to predict outcomes in adult patients undergoing cardiac surgery [3-5].
However, producing a risk prediction formula for thoracic surgeries has been more difficult. Procedures are less standardized than cardiac surgeries. Lung resection inevitably results in patient's physiologic deficit, unlike patients undergoing cardiac surgery who generally do not suffer post-operative physiologic deficit. This deficit is variable, according to the extent of lung resection, and the pre-existing lung function of the patient. Some patients receive preoperative chemotherapy and radiotherapy. Therefore, there have not been widely used risk scoring systems for thoracic surgery yet.
In this study, we tried to develop a risk scoring system for postoperative complications in thoracic surgical patients from the data between 2005 and 2007 in our hospital. We focused on prolonged ICU stay as the representative of postoperative complications because there exists a wide range of postoperative complications, but significant ones eventually lead to prolonged ICU stay. Usual patients return to the general ward on the following day or two. Thus, we regarded more than 3 days of ICU stay as prolonged ICU stay. We evaluated various possible risk factors in relation to prolonged ICU stay, and developed a risk scoring system by multiple logistic regression analysis.
We hope this study provides a stepping stone to developing a widely used risk scoring system in the field of thoracic surgery.
Materials and Methods
The hospital Institutional Review Board approved this study. Data from all patients who underwent major lung and esophageal cancer operations, between 2005 and 2007 in our hospital, were collected. Among them, patients who had complete data were analyzed (n = 858).
Surgery was contraindicated in those patients with a predicted post-operative forced expiratory volume in 1 second (ppoFEV1) and predicted a post-operative carbon monoxide lung diffusion capacity (ppoDLCO) of less than 30%. As a rule, lobectomies were performed through a standard posterolateral or an anterolateral muscle-sparing thoracotomy or video-assisted thoracic surgery. The same experienced surgeons (Y.S., J.K., K.K, Y.C., each of whom performs more than 100 major lung resection surgeries per year) conducted each operation. In the postoperative period, all patients were admitted to the ICU for a period of 24-48 h, and then transferred back to the thoracic ward. Post-operative treatment was standardized and focused on early mobilization, chest physiotherapy, physical rehabilitation, thoracotomy pain control, antibiotic and antithrombotic prophylaxis. All patients received postoperative pain control via epidural or continuous intravenous analgesia, which were titrated to keep the numeric pain rating score below 4 (in a scale ranging from 0 to 10) during the first post operative 48-72 h. The criteria of ICU discharge were no postoperative complications, stable vital signs, no desaturation (SpO2 < 90%), and less than 20% of heart rate increase on ambulation.
Patients' demographic, laboratory, anesthetic and surgical data were analyzed, in relation to more than 3 days of ICU stay. Univariate analysis was done for each risk factor candidates, and then multiple logistic regression analysis with forward stepwise method was done to find independent risk factors and subsequently to develop a risk scoring system. The odds ratio of each independent risk factors and area under the receiver operating characteristic (AUC) curve were obtained.
Statistical analysis
Discrete data are presented as percentages, and continuous data are presented as the mean ± SD. The student t test or Mann-Whitney test, with Bonferroni's correction, was used for continuous variables. The Pearson Chi-square or Fisher exact test was applied for categorical variables. Univariate and multiple logistic regression were performed for risk factors of prolonged ICU stay. All the statistical tests were two-tailed and a significance level of 0.05 was accepted. Statistical analysis was performed with the software program SAS 9.1.3 (SAS institute, Inc., Cary, NC, USA).
Results
Data from all patients who underwent major lung and esophageal cancer surgeries, between 2005 and 2007 in our hospital, were collected retrospectively. Among them, patients who had complete data were analyzed (n = 858).
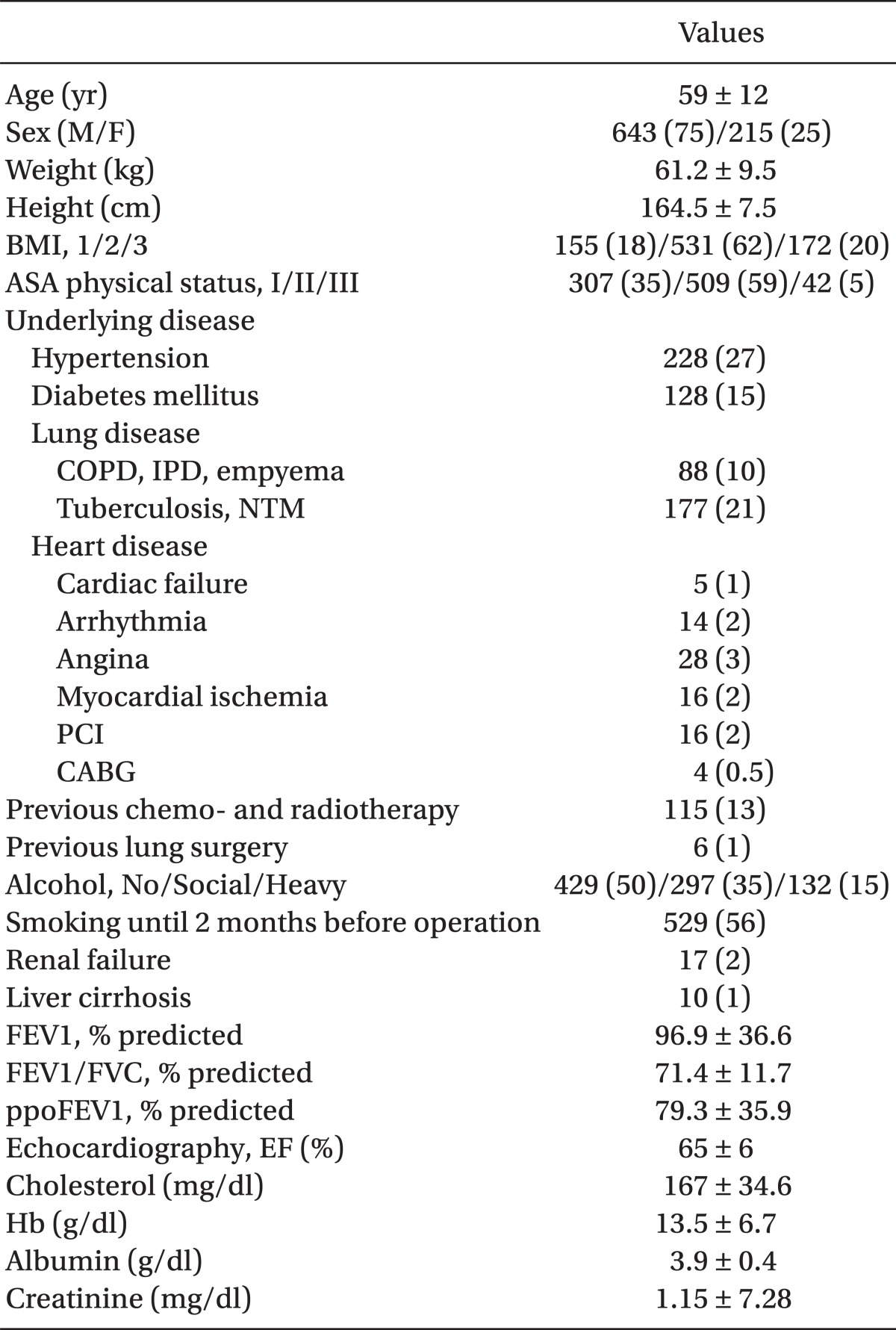
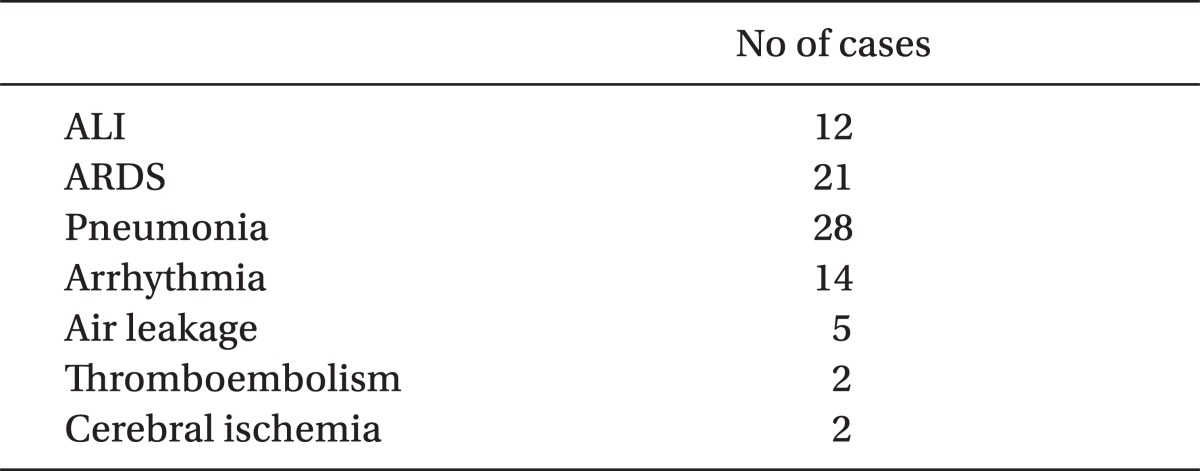
Demographic, laboratory and operative data were shown in Table 1 and 2. Mortality of present study was only 2%, during the 6 month follow-up and there was no mortality within 2 days of ICU stay (data was not shown). Seventy seven patients (9%) developed postoperative complications, which required more than 3 days of ICU stay. Lung complications comprised 72%, followed by arrhythmia (17%). The etiologies of prolonged stay are shown in Table 3.
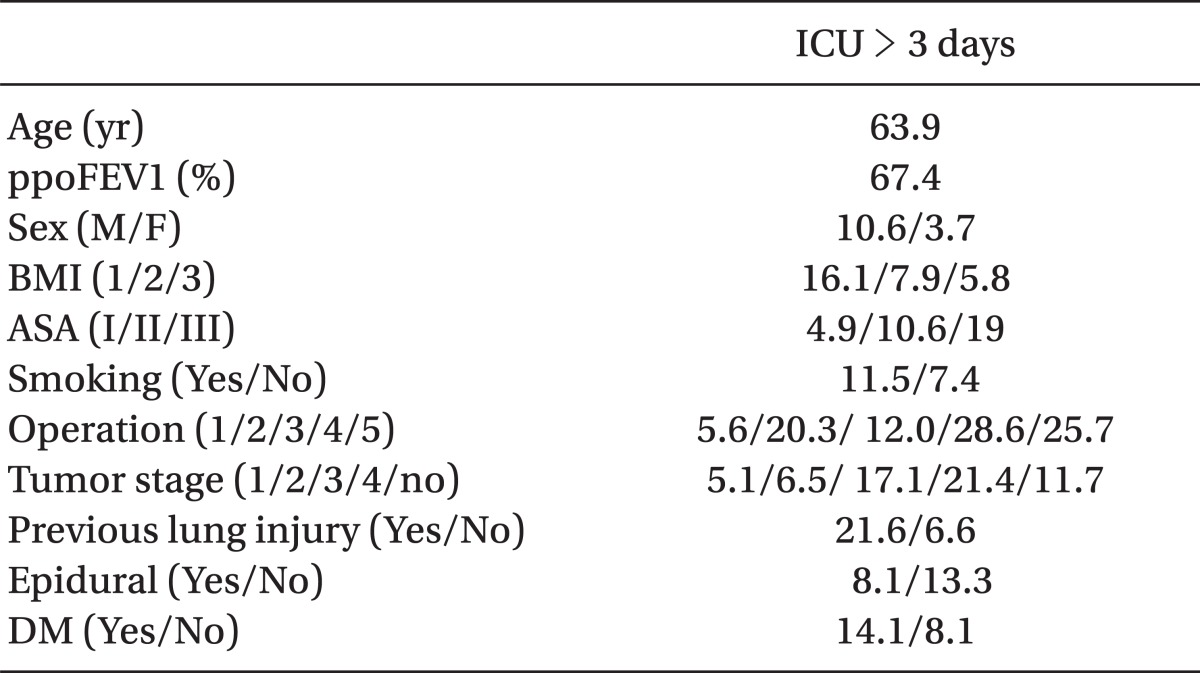
Age, sex, body mass index (BMI), American Society of Anesthesiologist (ASA) physical status classification, smoking, operation name, tumor stage, preoperative lung injury, no epidural analgesia, DM, and ppoFEV1 were the risk factors for prolonged ICU stay by a univariate analysis (Table 4).
Among them, age, operation name, preoperative lung injury, no epidural analgesia, and ppoFEV1 were the risk factors by multivariate analysis. The odds ratio of risk factors are shown in Table 5.
Table 6 summarized the risk score calculations for prolonged ICU stay (> 3 days). The risk score p for prolonged ICU stay was derived from the formula: logit(p/[1-p]) = -5.39 + 0.06 × age + 1.12 × operation name(2) + 1.52 × operation name(3) + 1.32 × operation name(4) + 1.56 × operation name(5) + 1.30 × preoperative lung injury + 0.72 × no epidural analgesia - 0.02 × ppoFEV1 [Age in years, operation name(2): pneumonectomy, operation name(3): esophageal cancer operation, operation name(4): completion pneumonectomy, operation name(5): extended operation, preoperative lung injury(+), epidural analgesia(-), ppoFEV1 in %. Extended operations: operation involved major vessels, trachea, pericardium, chest wall, diaphragm in addition to lung resection. Preoperative lung injury: idiopathic pulmonary fibrosis, bronchiectasis, chronic obstructive pulmonary disease (COPD), empyema, fungal lesion. Cases of ppoFEV1 < 30% were excluded in this formula. Patients who did not receive epidural analgesia received IV PCA].
According to this scoring system, a 73 year-old male with COPD and ppoFEV1 40% and who underwent a right lower lobe lobectomy, without epidural analgesia, has 35% possibility of prolonged ICU stay [logit(p/[1-p]) = -5.39 + 0.06 × 73 + 1.12 × 0 + 1.52 × 0 + 1.32 × 0 + 1.56 × 0 + 1.30 × 1 + 0.72 × 1 - 0.02 × 40 → P = 0.35].
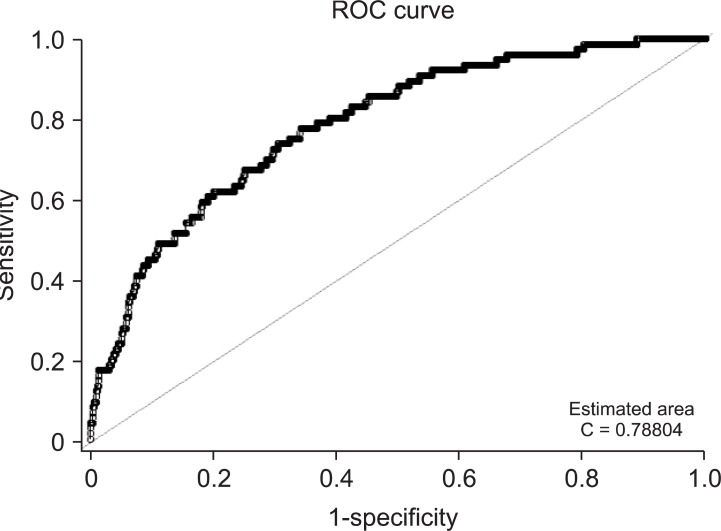
Area under the receiver operating characteristic (AUC) curve was 0.79 (95% CI 0.75-0.83), by this risk prediction model, which indicated that it is useful to identify surgical patients at higher risk for prolonged ICU stay (Fig. 1).
Discussion
In this study, we forged a risk scoring system which calculates the possibility of postoperative complications in patients undergoing major thoracic surgery.
Previous studies used mortality as an end point. However, mortality is low in thoracic surgeries these days, and obtaining mortality requires a large cohort and long period of follow-up. The overall mortality rates were 0.9% after 758 lung cancer surgeries [6]. Mortality of present study was 2% during a 6 month follow-up period (data was not shown). Postoperative complications are common in thoracic surgery and became a major concern. Therefore, we employed the incidence of postoperative complications as our end point. There can be various postoperative complications, but prolonged ICU stay, as the representative of postoperative complications, can lead us to include only the clinically significant complications. Seventy seven patients (9%) developed postoperative complications, which required prolonged ICU stay in this study. Lung complications comprised 72%, followed by arrhythmia (17%) for the etiology of prolonged ICU stay.
Risk scores better include small number of parameters for easy application. Further, rarely used tests should be avoided even if those tests can predict patients' risk more accurately. In this study, we only used age, operation name, presence of previous lung injury and epidural analgesia, and ppoFEV1, for our risk scoring system.
Two famous risk score systems for thoracic surgery have been published until now, by the European Thoracic Surgery Data base project [7]. Researchers collected data from 27 units in 14 countries to give a population of 3,426 patients undergoing first-time lung resection.
The European Societies Subjective Score (ESSSv1) presented ASA score, Medical Research Council dyspnea score, surgical procedure, and age as risk factors for in-hospital mortality. This score was named the "subjective" score as the risk factors ASA and dyspnea score were subjective and somewhat imprecise in nature [7].
The European Society Objective Score (ESOS.01) is a more objective score and only uses age and ppoFEV1 [7]. The risk formula was logit(p/[1-p]) = -5.8858 + 0.0501 × age - 0.0218 × ppoFEV1, to predict in-hospital mortality. This model was found to give a better fit of predicted and actual mortality than ESSSv1.
One single center study was conducted in 758 patients undergoing surgery for the treatment of non small cell lung cancer. Induction therapy and an extended surgical procedure were strong independent factors for the prediction of postoperative morbidity. Significant differences were also observed for pathologic stage, preoperative hemoglobin and carbon monoxide lung diffusion capacity (DLCO) levels, operation time, and intraoperative bleeding [6].
Therefore, our risk factors, age, operation name, presence of previous lung injury and ppoFEV1, are in line with previous studies, but were found to be more comprehensive. Epidural analgesia is a new factor. Reduction of morbidity by epidural catheter in thoracic surgery is a widely accepted notion [8].
We took ppoFEV1 as a parameter, and it is certainly the most widely used parameter in preoperative risk stratification and the measure is recommended by the British Thoracic Society (BTS) and American College of Chest Physicians (ACCP) functional guidelines as a first step in the screening process of patients for lung resection surgery [9]. One study suggested peak oxygen consumption (peakVO2) during Cardiopulmonary Exercise Test as a reliable predictor for complications and mortality in patients undergoing pulmonary lobectomy or pneumonectomy [10]. Reduced values of DLCO are recommended in other studies as a better parameter than ppoFEV1 [11,12]. Six minutes of walking and stair climbing also have been reported to be associated with postoperative complications and mortality [11]. However, peakVO2 is not routinely performed as a preoperative evaluation test in all patients before lung resection, and also, DLCO is still not routinely measured in all patients, but only in patients with airflow limitation [9,13]. Physiological functioning tests are not performed routinely either. Therefore, we took only ppoFEV1 as a preoperative laboratory test for the risk scoring system. However, we think other tests previously mentioned are good candidates for the inclusion of risk scoring system and should be conducted more often in practice to predict patient's risk better in the future.
We found sex, BMI, ASA classification, smoking, tumor stage, and diabetes mellitus were the risk factors for prolonged ICU stay by a univariate analysis (Table 4). Some factors are worth mentioning here, despite the lack of significance in multivariate analysis. ASA classification was a risk factor in ESSSv1. Our population has only 5% of ASA III patients and this may be the reason we were not able to show the correlation of ASA classification and prolonged ICU stay. Damaged lung, by smoking, can be evaluated by the factor of previous lung injury, which includes COPD, and smoking itself was not an independent risk factor by multivariate analysis. Interestingly, underweight patients (BMI1) showed more risk than normal or overweight patients in a univariate analysis. This finding was also proved by other report [14]. We assume that underweight patients may have less reserve to cope with postoperative physiological stress and have higher morbidity. Induction therapy was a risk factor in the previous study [6], but previous chemo- and radiotherapy was not a risk factor in our study. Previous study was published in 2005. These days, chemo- and radiotherapy may have improved to a degree where its influence on postoperative complications may be miniscule.
As for the limitations of our study, we used ppoFEV1, but as we previously stated, had all patients underwent DLCO or physiological functioning tests, it would have been better predictors, but in the absence of complete data (only 25% of patients had a preoperative measurement of DLCO, physiological functioning tests were conducted less frequently than DLCO), it was impossible to determine using the mentioned tests. Second, intrinsic bias and confounding of a cohort retrospective design and the single-center tertiary care nature of the institution are limitations. Third, it shares a common major limitation with similar scoring systems: intraoperative events are not taken into account. Adverse events, such as excessive bleeding and hypotension, may affect the postoperative outcome and skew scores, based solely on the preoperative variables. Forth, we included esophageal operation in this analysis because it comprised 16.5% of all thoracic operations in our hospital, and postoperative complications are relatively high. Esophageal operation has especially high pulmonary complications due to one lung ventilation, ischemia reperfusion, and systemic inflammatory reaction, in addition to surgical complications, such as anastomosis leakage. However, addition of esophageal operation may have resulted in some deviation on this scoring system [15]. Fifth, external validation is required, which will be done in a future study.
In conclusion, we provided a risk scoring system based on age, operation name, presence of previous lung injury and epidural catheter, and ppoFEV1 for postoperative complications in thoracic surgery patients. We hope that this study may provide a stepping stone in developing a widely used risk scoring system in thoracic surgery.