2. Perondi MB, Reis AG, Paiva EF, Nadkarni VM, Berg RA. A comparison of high-dose and standard-dose epinephrine in children with cardiac arrest. N Engl J Med 2004; 350: 1722-1730. PMID:
15102998.


3. Todres ID, Fugate JH. Critical Care for infants and children. 1996, Newyork, Little Brown & Co.
4. Goetting MG, Paradis NA. High-dose epinephrine improves outcome from pediatric arrest. Ann Emerg Med 1991; 20: 22-26. PMID:
1984722.


5. Bj├Ėrshol CA, S├Ėreide E, Torsteinb├Ė TH, Lexow K, Nilsen OB, Sunde K. Quality of chest compressions during 10 min of single-rescuer basic life support with different compression: ventilation ratios in a manikin model. Resuscitation 2008; 77: 95-100. PMID:
18207627.


6. Vento M, Saugstad OD. Resuscitation of the term and preterm infant. Semin Fetal Neonatal Med 2010; 15: 216-222. PMID:
20451481.


7. Dalens B. Regional anesthesia in pediatrics. Ann Fr Anesth Reanim 1989; 8: 51-66. PMID:
2653120.


8. Thomas JM, Schug SA. Recent advances in the pharmacokinetics of local anesthetics. Long-acting amide enantiomers and continuous infusion. Clin Pharmacokinet 1999; 36: 67-83. PMID:
9989343.


9. Karmakar MK, Kwok WH. Edited by Cot├® CJ, Lerman J, Todres DUltrasound-Guided Regional Anesthesia. A Practice of Anesthesia for Infants and Children. 2009, 4th ed. : Philadelphia, Saunders Elsevier. pp 911-938.

10. Flandin-Bl├®ty C, Barrier G. Accidents following extradural anesthesia in children. The results of a retrospective study. Paediatr Anaesth 1995; 5: 41-46. PMID:
8521309.


11. Giaufre E, Dalens B, Gombert A. Epidemiology and morbidity of regional anesthesia in children: a one-year prospective survey of the French-Language Society of Pediatric Anesthesiologists. Anesth Analg 1996; 83: 904-912. PMID:
8895261.


13. Tsui BC, Suresh S. Ultrasound imaging for regional anesthesia in infants, children and adolescents: a review of current literature and its application in the practice of neuraxial blocks. Anesthesiology 2010; 112: 719-728. PMID:
20179511.


14. Eger EI 2nd, Saidman LJ, Brandstater B. Minimum alveolar anesthetic concentration: a standard of anesthetic potency. Anesthesiology 1965; 26: 756-763. PMID:
5844267.


15. Paul M, Fisher DM. Are estimates of MAC reliable? Anesthesiology 2001; 95: 1362-1370. PMID:
11748393.


16. Cot├® CJ, Lerman J, Ward RM, Lugo RA, Goudsouzian N. Edited by Cot├® CJ, Lerman J, Todres DPharmacokinetics and Pharmakology of Drugs Used in Children. A Practice of Anesthesia for Infants and Children. 2009, 4th ed. : Philadelphia, Saunders Elsevier. pp 100-119.
17. Maruyama K, Agata H, Ono K, Hiroki K, Fujihara T. Slow induction with sevoflurane was associated with complete atrioventricular block in a child with hypertension, renal dysfunction, and impaired cardiac conduction. Paediatr Anaesth 1998; 8: 73-78. PMID:
9483603.


18. Driessen JJ, van Oort AM, Booij LH. Severe myocardial ischaemia during mask induction of anaesthesia in an infant with unknown critical supravalvular aortic stenosis. Anaesthesia 2003; 58: 568-570. PMID:
12846623.


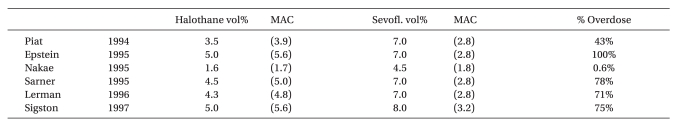
19. Piat V, Dubois MC, Johanet S, Murat I. Induction and recovery characteristics and hemodynamic responses to sevoflurane and halothane in children. Anesth Analg 1994; 79: 840-844. PMID:
7978397.


20. Epstein RH, Stein AL, Marr AT, Lessin JB. High concentration versus incremental induction of anesthesia with sevoflurane in children: a comparison of induction times, vital signs, and complications. J Clin Anesth 1998; 10: 41-45. PMID:
9526937.


21. Nakae Y, Miyabe M, Sonoda H, Kawana S, Namiki A. Comparison of intubating condition under sevoflurane and halothane anesthesia in pediatric patients. Masui 1995; 44: 239-243. PMID:
7739097.

22. Sarner JB, Levine M, Davis PJ, Lerman J, Cook DR, Motoyama EK. Clinical characteristics of sevoflurane in children: a comparison with halothane. Anesthesiology 1995; 82: 38-46. PMID:
7832332.


23. Lerman J, Davis PJ, Welborn LG, Orr RJ, Rabb M, Carpenter R, et al. Induction, recovery, and safety characteristics of sevoflurane in children undergoing ambulatory surgery: a comparison with halothane. Anesthesiology 1996; 84: 1332-1340. PMID:
8669674.


24. Sigston PE, Jenkins AM, Jackson EA, Sury MR, Mackersie AM, Hatch DJ. Rapid inhalation induction in children: 8% sevoflurane compared with 5% halothane. Br J Anaesth 1997; 78: 362-365. PMID:
9135351.



25. Holzki J, Kretz FJ. Changing aspects of sevoflurane in paediatric anaesthesia: 1975-99. Paediatr Anaesth 1999; 9: 283-286. PMID:
10411761.


26. Cot├® CJ, Lerman J, Ward RM, Lugo RA, Goudsouzian N. Edited by Cot├® CJ, Lerman J, Todres IDPharmacokinetics and Pharmakology of Drugs Used in Children. A Practice of Anesthesia for Infants and Children. 2009, 4th ed. : Philadelphia, Saunders Elsevier. p 105.
27. Bhananker SM, Ramamoorthy C, Geiduschek JM, Posner KL, Domino KB, Haberkern CM, et al. Anesthesia-related cardiac arrest in children: update from the Pediatric Perioperative Cardiac Arrest Registry. Anesth Analg 2007; 105: 344-350. PMID:
17646488.


28. Paris ST, Cafferkey M, Tarling M, Hancock P, Yate PM, Flynn PJ. Comparison of sevoflurane and halothane for outpatient dental anaesthesia in children. Br J Anaesth 1997; 79: 280-284. PMID:
9389840.



29. Cot├® CJ, Lerman J, Ward RM, Lugo RA, Goudsouzian N. Edited by Cot├® CJ, Lerman J, Todres IDPharmacokinetics and Pharmakology of Drugs Used in Children. A Practice of Anesthesia for Infants and Children. 2009, 4th ed. : Philadelphia, Saunders Elsevier. p 108.
30. Wodey E, Pladys P, Copin C, Lucas MM, Chaumont A, Carre P, et al. Comparative hemodynamic depression of sevoflurane versus halothane in infants: an echcardiographic study. Anesthesiology 1997; 87: 795-800. PMID:
9357880.


31. Haga S, Shima T, Momose K, Andoh K, Hashimoto Y. Anesthetic induction of children with high concentrations of sevoflurane. Masui 1992; 41: 1951-1955. PMID:
1479663.

32. Yli-Hankala A, Vakkuri A, S├żrkel├ż M, Lindgren L, Korttila K, J├żntti V. Epileptiform electroencephalogram during mask induction of anesthesia with sevoflurane. Anesthesiology 1999; 91: 1596-1603. PMID:
10598599.


33. Kaisti KK, J├ż├żskel├żinen SK, Rinne JO, Mets├żhonkala L, Scheinin H. Epileptiform discharges during 2 MAC sevoflurane anesthesia in two healthy volunteers. Anesthesiology 1999; 91: 1952-1955. PMID:
10598642.


34. Vakkuri A, Yli-Hankala A, S├żrkel├ż M, Lindgren L, Mennander S, Korttila K, et al. Sevoflurane mask induction of anaesthesia is associated with epileptiform EEG in children. Acta Anaesthesiol Scand 2001; 45: 805-811. PMID:
11472278.


35. J├ż├żskel├żinen SK, Kaisti K, Suni L, Hinkka S, Scheinin H. Sevoflurane is epileptogenic in healthy subjects at surgical levels of anesthesia. Neurology 2003; 61: 1073-1078. PMID:
14581667.


36. Constant I, Seeman R, Murat I. Sevoflurane and epileptiform EEG changes. Paediatr Anaesth 2005; 15: 266-274. PMID:
15787916.


37. S├żrkel├ż MO, Ermes MJ, van Gils MJ, Yli-Hankala AM, J├żntti VH, Vakkuri AP. Quantification of epileptiform electroencephalographic activity during sevoflurane mask induction. Anesthesiology 2007; 107: 928-938. PMID:
18043061.


38. Voss LJ, Sleigh JW, Barnard JP, Kirsch HE. The howling cortex: seizures and general anesthetic drugs. Anesth Analg 2008; 107: 1689-1703. PMID:
18931234.


39. Shichinohe Y, Masuda Y, Takahashi H, Kotaki M, Omote T, Shichinohe M, et al. A case of postoperative hepatic injury after sevoflurane anesthesia. Masui 1992; 41: 1802-1805. PMID:
1460759.

40. Iwanaga Y, Komatsu H, Yokono S, Ogli K. Serum glutathione S-transferase alpha as a measure of hepatocellular function following prolonged anaesthesia with sevoflurane and halothane in paediatric patients. Paediatr Anaesth 2000; 10: 395-398. PMID:
10886696.


41. Singhal S, Gray T, Guzman G, Verma A, Anand K. Sevoflurane hepatotoxicity: a case report of sevoflurane hepatic necrosis and review of the literature. Am J Ther 2010; 17: 219-222. PMID:
19455019.


42. B├Čsenberg AT, Murat I. Edited by Cot├® CJ, Lerman J, Todres DPediatric anesthesia in developing countries. A Practice of Anesthesia for Infants and Children. 2009, 4th ed. : Philadelphia, Saunders Elsevier. pp 1077-1084.

43. Cravero J, Surgenor S, Whalen K. Emergence agitation in paediatric patients after sevoflurane anaesthesia and no surgery: a comparison with halothane. Paediatr Anaesth 2000; 10: 419-424. PMID:
10886700.


44. Russell IA, Miller Hance WC, Gregory G, Balea MC, Cassorla L, DeSilva A, et al. The safety and efficacy of sevoflurane anesthesia in infants and children with congenital heart disease. Anesth Analg 2001; 92: 1152-1158. PMID:
11323338.


45. Cohen IT, Hannallah RS, Hummer KA. The incidenceof emergence agitation associated with deflurane anesthesia in children. Anesth Analg 2001; 93: 88-91. PMID:
11429345.


46. Lerman J, Hammer GB, Verghese S, Ehlers M, Khalil SN, Betts E, et al. Airway responses to desflurane during maintenance of anesthesia and recovery in children with laryngeal mask airways. Paediatr Anaesth 2010; 20: 495-505. PMID:
20456065.


47. Rosow C. Remifentanil: a unique opioid analgesic. Anesthesiology 1993; 79: 875-876. PMID:
7902031.

48. Akpek EA, Erkaya C, Donmez A, Mercan S, Esen A, Aslamaci S, et al. Remifentanil use in children undergoing congenital heart surgery for left-to-right shunt lesions. J Cardiothorac Vasc Anesth 2005; 19: 60-66. PMID:
15747271.


49. Ross AK, Davis PJ, Dear Gd GL, Ginsberg B, McGowan FX, Stiller RD, et al. Pharmacokinetics of remifentanil in anesthetized pediatric patients undergoing elective surgery or diagnostic procedures. Anesth Analg 2001; 93: 1393-1401. PMID:
11726413.


50. Crawford MW, Hayes J, Tan JM. Dose-response of remifentanil for tracheal intubation in infants. Anesth Analg 2005; 100: 1599-1604. PMID:
15920180.


51. Choong K, AlFaleh K, Doucette J, Gray S, Rich B, Verhey L, et al. Remifentanil for endotracheal intubation in neonates: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed 2010; 95: F80-F84. PMID:
20231228.


52. Vinik HR, Kissin I. Rapid development of tolerance to analgesia during remifentanil infusion in humans. Anesth Analg 1998; 86: 1307-1311. PMID:
9620525.


53. Blair JM, Hill DA, Wilson CM, Fee JP. Assessment of tracheal intubation in children after induction with propofol and different doses of remifentanil. Anaesthesia 2004; 59: 27-33. PMID:
14687095.


54. Nafiu OO, Kheterpal S, Morris M, Reynolds PI, Malviya S, Tremper KK. Incidence and risk factors for preincision hypotension in a noncardiac pediatric surgical population. Paediatr Anaesth 2009; 19: 232-239. PMID:
19143955.


55. Kanto J, Gepts E. Pharmacokinetic implications for the clinical use of propofol. Clin Pharmacokinet 1989; 17: 308-326. PMID:
2684471.


56. Holzki J, Aring C, Gillor A. Death after re-exposure to propofol in a 3-year-old child: a case report. Paediatr Anaesth 2004; 14: 265-270. PMID:
14996268.


57. Kill C, Leonhardt A, Wulf H. Lacticacidosis after short-term infusion of propofol for anaesthesia in a child with osteogenesis imperfect. Paediatr Anaesth 2003; 13: 823-826. PMID:
14617125.


58. Chukwuemeka A, Ko R, Ralph-Edwards A. Short-term low-dose propofol anaesthesia associated with severe metabolic acidosis. Anaesth Intensive Care 2006; 34: 651-655. PMID:
17061643.


59. Laquay N, Prieur S, Greff B, Meyer P, Orliaguet G. Propofol infusion syndrome. Ann Fr Anesth Reanim 2010; 29: 377-386. PMID:
20399595.


60. Ivani G, De Negri P, Conio A, Amati M, Roero S, Giannone S, et al. Ropivcacaine-clonidine combination for caudal blockade in children. Acta Anaesthesiol Scand 2000; 44: 446-449. PMID:
10757579.


61. Sillanp├ż├ż M. Clonidine prophylaxis of childhood migraine and other vascular headache. a double blinded study of 57 children;. Headache 1977; 17: 28-31. PMID:
321395.


62. Tobias JD, Berkenbosch JW. Initial experience with dexmedetomidine in paediatric-aged patients. Paediatr Anaesth 2002; 12: 171-175. PMID:
11882231.


63. Bhana N, Goa KL, McClellan KJ. Dexmedetomidine. Drugs 2000; 59: 263-268. PMID:
10730549.


64. Vilo S, Rautiainen P, Kaisti K, Aantaa R, Scheinin M, Manner T, et al. Pharmacokinetics of intravenous dexmedetomidine in children under 11 yr of age. Br J Anaesth 2008; 100: 697-700. PMID:
18378546.



65. Potts AL, Larsson P, Eksborg S, Warman G, L├Čnnqvist PA, Anderson BJ. Clonidine disposition in children; a population analysis. Paediatr Anaesth 2007; 17: 924-933. PMID:
17767627.


66. Petroz GC, Sikich N, James M, van Dyk H, Shafer SL, Schily M, et al. A phase I, two-center study of the pharmacokinetics and pharmacodynamics of dexmedetomidine in children. Anesthesiology 2006; 105: 1098-1110. PMID:
17122572.


67. Tobin JR, Shafer SL, Davis PJ. Pediatric research and scholarship: another Gordian knot? Anesth Analg 2006; 103: 43-48. PMID:
16790623.


68. Fisher D. Do the right thing (or Do the market exclusivity thing?). Anesthesiology 2006; 105: 1074-1075. PMID:
17122566.


69. Sorgenfrei IF, Norrild K, Larsen PB, Stensballe J, Ostergaard D, Prins ME, et al. Reversal of rocuronium-induced neuromuscular block by the selective relaxant binding agent sugammadex: a dose-finding and safety study. Anesthesiology 2006; 104: 667-674. PMID:
16571960.


70. Khine HH, Corddry DH, Kettrick RG, Martin TM, McCloskey JJ, Rose JB, et al. Comparion of cuffed and uncuffed endotracheal tubes in young children during general anesthesia. Anesthesiology 1997; 86: 627-631. PMID:
9066329.


71. Fisher DM. Highlight: comparison of cuffed and uncuffed endotracheal tubes in young children during general anesthesia. Anesthesiology 1997; 86V: 27A.
72. Newth CJ, Rachman B, Patel N, Hammer J. The use of cuffed vs uncuffed endotracheal tubes in intensive care. J Pediatr 2004; 144: 333-337. PMID:
15001938.


73. Deakers TW, Reynolds G, Stretton M, Newth CJ. Cuffed endotracheal tubes in pediatric intensive care. J Pediatr 1994; 125: 57-62. PMID:
8021785.


74. Dubreuil C. Laryngotracheal stenosis in children. Pediatrie 1987; 42: 273-279. PMID:
3313266.

75. Wiel E, Vilette B, Darras JA, Scherpereel P, Leclerc F. Larngotracheal stenosis in children after intubation. Report of five cases. Paediatr Anaesth 1997; 7: 415-419. PMID:
9308067.


76. Ashtekar CS, Wardhaugh A. Do cuffed endotracheal tubes increase the risk of airway mucosal injury and post-extubation stridor in children? Arch Dis Child 2005; 90: 1198-1199. PMID:
16243883.



77. Holzki J, Laschat M, Puder C. Stridor is not a scientifically valid outcome measure for assessing airway injury. Paediatr Anaesth 2009; 19(Suppl 1): 180-197. PMID:
19572855.


78. Holzki J, Laschat M, Puder C. Iatrogenic damage to the pediatric airway. Mechanisms and scar development. Paediatr Anaesth 2009; 19(Suppl 1): 131-146. PMID:
19572852.


79. Weiss M, Dullenkopf A, Fischer JE, Keller C, Gerber AC. European Paediatric Endotracheal Intubation Study Group. Prospective randomized controlled multi-centre trial of cuffed or uncuffed endotracheal tubes in small children. Br J Anaesth 2009; 103: 867-873. PMID:
19887533.