Long QT syndrome provoked by induction of general anesthesia -A case report-
Article information
Abstract
Long QT syndrome (LQTS) is an arrhythmogenic cardiovascular disorder resulting from mutations in cardiac ion channels. LQTS is characterized by prolonged ventricular repolarization and frequently manifests itself as QT interval prolongation on the electrocardiogram (ECG). A variety of commonly prescribed anesthetic drugs possess the adverse property of prolonging cardiac repolarization and may provoke serious ventricular tachyarrhythmia called 'torsades de pointes', ventricular fibrillation, and sudden death. We experienced a case of ventricular tachycardia and ventricular fibrillation after anesthetic induction and it came out into the open that anesthetic induction provoked long QT syndrome.
Long QT syndrome (LQTS) is a cardiovascular disorder characterized by prolonged ventricular repolarization and prolongation of the QT interval on the electrocardiogram (ECG), which may occasionally cause serious arrhythmias leading to sudden cardiac death. Routinely, patients admitted for operation are required to take a variety of prescription drugs, some of which can provoke negative effects in patients by prolonging cardiac repolarization. Medications administered during general anesthesia are known to influence QT intervals [1], and such drug-induced QT prolongation may increase the risk of ventricular arrhythmias in patients with LQTS which can be fatal.
We witnessed a case diagnosed with LQTS following ventricular fibrillation after the induction of general anesthesia in a patient, for open reduction of fracture of the zygomatic bone on the right side. We report our clinical experience and review the relevant literature.
Case Report
A 32-year-old, 173 cm, 79 kg male patient was admitted to the emergency room (ER) for the treatment of facial pain due to an automobile accident. On arrival, he was fully conscious with an Glasgow Coma Scale score of 15/15 and stable vital signs: blood pressure (BP) 115/76 mmHg, heart rate (HR) 58 beats/min, and body temperature 37℃. Frontal skull fracture was suspected, and an X-ray skull and computed tomography (CT) were taken which demonstrated a fracture of the zygomatic bone on the right side. Under general anesthesia, an open reduction of the right sided zygomatic bone fracture was planned. Patient's medical history, neurological and physical examinations were normal. Preoperative blood test findings were normal: Na+ 143 mEq/L, K+ 3.9 mEq/L, Cl- 106 mEq/L, Ca2+ 9.3 mg/dl. The electrocardiogram (ECG) and chest X-ray results were also normal (Fig. 1). No premedication was provided, and the patient's vital signs were measured immediately after entering the operating room (OR); BP was 120/80 mmHg, HR was 50 beats/min, and pulse oximetry (SpO2) was 99%. Due to the facial injury, facemask ventilation was thought to be difficult to perform and hence, manual assisted ventilation, using rapid-sequence induction, with O2 of 5 L/min was performed and, 450 mg of thiopental and 80 mg of succinylcholine were injected intravenously one after another. After tracheal intubation, manual assisted ventilation was performed with 2.0 vol% of sevoflurane.
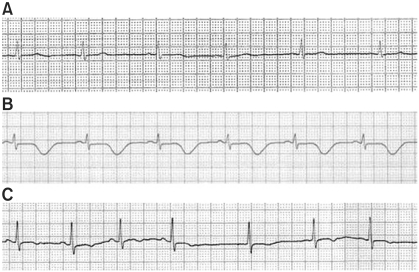
Electrocardiography in the lead II rhythm strip, (A) before induction (55 BPM, QTc = 439 ms), (B) after induction at ICU (59 BPM, QTC = 547 ms), (C) before reoperation (68 BPM, QTC = 406 ms).
Immediately after tracheal intubation, BP increased from 120/80 to 160/90 mmHg and HR rose from 50 to 120 beats/min, and premature ventricular complexes (PVC) were detected on ECG. Therapy for ventricular arrthymias was intitated with a 70 mg of lidocaine intravenous injection, but the ventricular arrhythmias changed into patterns of ventricular bigeminy, ventricular tachycardia (VT), and torsades de pointes subsequently; BP measured at that time was 60/30 mmHg. Immediately, the administration of sevoflurane was discontinued and manual assisted ventilation was performed with 100% O2, while an external cardiac defibrillator was being prepared for attempting defibrillation. As an intravenous injection of esmolol was being prepared, ventricular fibrillation (VF) without measurable BP was detected. Instantly, cardiopulmonary resuscitation (CPR) by chest compression was performed. As early defibrillation was attempted using 360 J, chest compression was resumed and 1 mg of epinephrine was administered intravenously. Thereafter, VF on ECG disappeared, and the heart rhythm returned to its normal sinus rhythm (NSR). Considering it difficult to proceed with the operation, the patient was transferred to the intensive care unit (ICU) as soon as the patient's vital signs stabilized and normal spontaneous respiration was confirmed.
After the patient was transferred to the ICU, arterial blood gas analysis (ABGA) and electrolyte studies were conducted and the results showed a pH of 7.334, PaCO2 of 44.8 mmHg, PaO2 of 70.1 mmHg, base excess -2.7 mmol/L, HCO3- 23.3 mmol/L, SaO2 of 93%, Na+ 142 mmol/L, K+ 4.2 mmol/L, Cl- 103 mmol/L, Ca2+ 1.16 mmol/L, and Mg2+ 1.7 mg/dl; which were within their normal limits. However, chest X-ray revealed cardiomegaly and mild pulmonary edema, while ECG detected a T-wave inversion in leads II, III, aVF, and V2-6, and QTc prolongation to 547 ms (Fig. 1). On ultrasound, akinesia of the anteroseptum & inferoposterolateral wall of the left ventricle was detected, and in coronary angiography, the left ventricular ejection fraction was 38% demonstrating hyposystole, and an increase in the left ventricular cavity size to 60 mm were detected, while there were no other abnormalities seen. Thereafter, ECG was recorded daily on a continous basis with Holter monitor. Epinephrine provocation test was positive and hence the patient was diagnosed with LQTS. On the fifth day of admission, implantable cardioverter-defibrillator (ICD) was implanted. Afterwards, the patient's vital signs improved and were within their normal ranges and the QTc intervals on ECG also showed normalization. Hence on the twelfth day of admission, open reduction of the fractured zygomatic bone on the right side was performed under general anesthesia.
In the preoperative evaluation, blood tests showed findings within their normal ranges. On ECG, T-wave inversion persisted in leads II, III, and aVF. Normal T-waves were seen in V2-6 and the QTc of 406 ms was also within its normal limits (Fig. 1). As a premedication, 2.5 mg of midazolam was injected intravenously at the entrance of the OR. On arrival in the OR, vital signs were measured: BP 110/75 mmHg; HR 85 beats/min; SpO2 95%. Before induction of anesthesia, preoxygenation was performed with 6 L/min of O2, and propofol administration was initiated with an effect-site concentration of 4.0 µg/ml using a target-controlled infusion device (Orchestra™, Fresenius Vial S.A, France). Afterwards, 0.15 µg/kg/min of remifentanil, a useful opioid, was continuously injected intravenously, loss of consciousness was confirmed. Following complete muscle relaxation by an intravenous injection of 70 mg of rocuronium, tracheal intubation was performed. BP recorded immediately after intubation was 100/60 mmHg, and HR was 75 beats/min. After the patient's vital signs stabilized, propofol was infused with an effect-site concentration of 2.5-3.0 µg/ml, and continuous remifentanil infusion was given at a rate of 0.05 µg/kg/min to 0.10 µg/kg/min. During anesthesia, no particular changes were noted in BP and on ECG. The left radial artery was catheterized to monitor the arterial pressure. Similarly, HR, ECG, SpO2, end-tidal CO2, etc. were monitored, but they did not reveal any particular changes. After completion of the operation, muscle relaxation was reversed immediately with an infusion of glycopyrrolate 0.2 mg and pyridostigmine 10 mg. After confirming normal spontaneous respiration and consciousness, the patient was extubated and when his vital signs were found to be stable, he was transferred to the recovery room (RR). The total time exposed to anesthesia was 3 hours and 45 minutes. In the RR, the patient was in a stable condition and after he fully recovered from the anesthesia, he was transferred to the general ward. On the nineth postoperative day, the patient was discharged without any complications.
Discussion
LQTS is an inherited or acquired disorder, but is usually inherited, which may cause sudden death due to episodes of syncope, seizure, and ventricular tachycardia. It is a rare disorder, with an estimated prevalence of around 1 per 2,500-10,000 [2]. In Korea, a study was performed by Koo et al. [3] on anesthetic management of a patient with congenital LQTS. It has been known that LQTS is caused by mutations in 1 of the 12 genes and, these mutations may create abnormalities in cardiac ion channels [4], prolong ventricular repolarization, and prolong the QT interval, causing serious arrhythmias such as ventricular tachycardia or torsades de pointes. In general, a corrected QT (QTc) interval is considered prolonged if the interval is longer than 440 ms. The LQTS diagnostic criteria proposed by Schwartz et al. [5], are based on ECG findings, clinical history of syncope or congenital deafness, and family history. Difinite LQTS is difined by the LQTS score reaches 4 or more points (Table 1). However, it is difficult to diagnose LQTS accurately only on the basis of the clinical history and ECG findings because of variable penetrance and genetic heterogeneity. Therefore, genetic testing is recommended [6], but diagnostic genotyping is very expensive and hence it is not practically feasible. Furthermore, 30% of patients with congenital LQTS have apparently normal phenotype and thus a normal QT interval, so they remain undiagnosed until an initiating event [7]. In addition, a variety of commonly prescribed drugs given to patients can prolong cardiac repolarization, prolong the QTc. These patients are considered as potential or silent LQTS gene carriers and 70% of them have a normal QTc interval until they are exposed to a provoking drug [8].
In the present case at the first attempt for open reduction, there were no preoperative abnormal findings in the patient's clinical history and family history, and the QTc on preoperative ECG was 439 ms, which can bearly be considered as prolonged QTc. Also, Per the LQTS score was 0; hence it was difficult to term the patient as a LQTS case and was a low risk case. However, when the medication for anesthetic induction was administered and tracheal intubation was performed, ventricular tachycardia and fibrillation did occur. After defibrillation, the patients vital signs stabilized. Later, based on the findings on ECG taken in the ICU which revealed that the QTc was prolonged to 547 ms and T-wave inversion was detected in leads II, III, aVF, and V2-6, the patient was diagnosed with LQTS. Therefore, it seems plausible to assume that he was an asymptomatic potential LQTS patient and the medication administered for anesthetic induction triggered LQTS.
Even though from many studies, the effects of anesthetic drugs on QT interval have been known, it is difficult to identify which particular medication is responsible for the QT interval prolongation as several drugs are administered simultaneously. To date, there has been no study reporting the usage of only one single anesthetic drug to induce anesthesia and investigating its effect on the QTc interval. In this case for rapid-sequence induction, thiopental and succinylcholine were injected, while 2.0 vol% of sevoflurane was also administered shortly. According to Saarnivaara and Lindgren [9], thiopental prolongs the QTc in the healthy patients and succinylcholine is effective in maintaining the QTc interval prolongation. Yildirim et al. [10] have reported that sevoflurane, isoflurane, and desflurane all prolong the QTc and there is a possibility that all the medications administered for anesthetic induction prolong the QTc. As sudden sympathetic activation due to tracheal intubation may also be a cause of prolongation of the QTc; Galloway and Glass [11] have suggested that drugs which activate the sympathetic nervous system; such as ketamine and pancuronium, should be avoided.
Magnesium sulphate is the treatment of choice in LQTS patients when an arrhythmic episode such as torsades de pointes develops. If magnesium sulphate is ineffective in suppressing torsades de pointes, temporary transvenous pacing is a viable alternative and it is recommended to apply transvenous pacing to the right atrium through the central venous catheter. In the present case, we did not have time for these options due to the transition into VF, and hence, defibrillation had to be attempted promptly. The use of drugs such as epineprine and norepinephrine that activate the sympathetic nervous system may lead to further prolongation of QTc interval. In this case wherein the patient had VF and cardiac arrest without measurable BP, we however had to administer epinephrine while performing CPR by chest compression.
The purpose of the treatment for LQTS is to prevent torsades de pointes and sudden cardiac death. Generally, beta-receptor blocking agents (β-blockers) are the first choice in the treatment of LQTS. Recently in the cases of episodes of syncope, torsades de pointes, or cardiac arrest requiring CPR despite the administration of β-blockers, ICD implantation is being performed. ICD implantation is also recommended for patients with prolonged QTc to 550-600 ms or more; dangerous enough for leading to sudden cardiac death [12]. Even though ICD does not prevent torsades de pointes, it is known that ICD reduces the risk of torsades de pointes or sudden cardiac death due to VF [13]. In the present case, because the patient had a prolonged QTc of 547 ms and had a history of cardiac arrest accompanied with VF, ICD implantation was performed and HR was well maintained at 70-80 beats/min.
Patients with LQTS require a careful anesthetic management. Preoperatively, β-blockers should be administered; HR should maintained below 130 beats/min; normal serum electrolyte levels should be maintained. It is recommended to avoid any medical agents that have the potential to prolong the QT interval. It is also important to pay attention to sudden activation of the sympathetic nervous system, and ICD needs to be evaluated. In the preoperative assessment of the present case, the patient did not complain of any particular symptoms, and the blood test findings including the serum electrolyte levels were normal. In the present case at the second attempt for open reduction, in the preoperative ECG evaluation, inverted T-waves in lead V2-6 detected on ECG taken in the ICU were normalized; the QTc was in the normal range; HR was well maintained to 70-80 beats/min. Therefore, β-blockers were not used and the operation was started after ICD was evaluated. It has been reported that inhalation anesthetics such as sevoflurane, isoflurane, and desflurane may prolong the QTc [10] and Michaloudis et al. [14] have suggested that the propofol does not affect the QTc interval much, while midazolam does not influence the QTc interval either. In light of these suggestions, in order to allay the patient's fear, we also gave intravenous administration of midazolam as a premedication to prevent sympathetic stimulation due to tracheal intubation and the operation and to minimize the influence on the QTc interval, we performed total intravenous anesthesia using mainly propofol and remifentanil, which was well maintained without any significant changes on vital signs and ECG. Recent research has identified that atropine and glycopyrrolate can prolong the QT interval [15]. Hence, it is safe to avoid the reversal of muscle relaxation as far as possible. In the present case, however, BP and HR were very well maintained and muscle relaxation reversal was performed, and it did not lead to any problems.
In conclusion, our patient may be a potential LQTS gene carrier, and was diagnosed with LQTS after he was exposed to the provoking anesthesia-inducing drugs, which precipitated the malignant arrhythmia. A variety of commonly administered drugs for general anesthesia can affect the QT interval and cause unexpected life-threatening situations. It is beneficial to make a proper choice of anesthetic drugs which do not affect the QT interval for both; the patients diagnosed with LQTS and the patients not diagnosed with LQTS, as a patient not diagnosed with LQTS may also be a potential LQTS patient and exposure to a provoking drug can make such a patient transit to an LQTS phase. Needless to say, that more attention should be directed to the anesthetic management of patients diagnosed with LQTS.
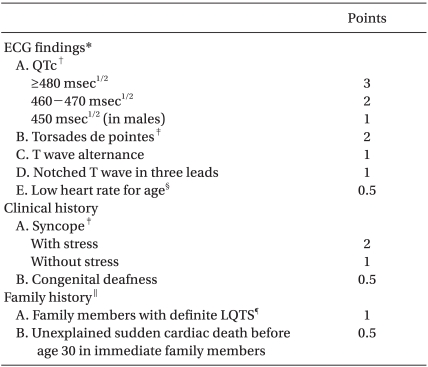
 ‡Mutually exclusive. §Resting heart rate below the second percentile for age. ∥The same family member cannot be counted in A and B. ¶Definite LQTS is defined by a LQTS score ≥4. Scoring: ≤1 point, low probability of LQTS; 2 to 3 points, intermediate probability of LQTS; ≥4 points, high probability of LQTS.
‡Mutually exclusive. §Resting heart rate below the second percentile for age. ∥The same family member cannot be counted in A and B. ¶Definite LQTS is defined by a LQTS score ≥4. Scoring: ≤1 point, low probability of LQTS; 2 to 3 points, intermediate probability of LQTS; ≥4 points, high probability of LQTS.