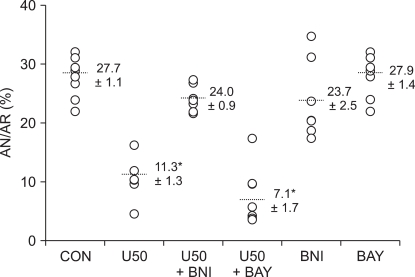
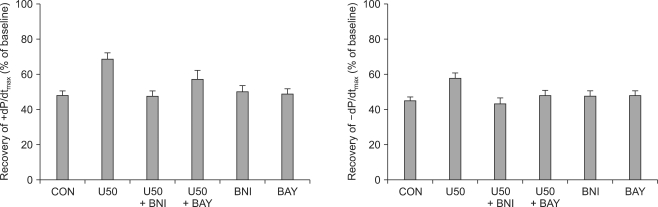
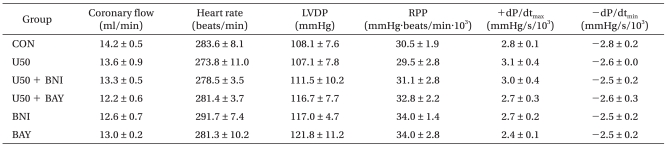
1. Lee YC, Jang YH, Kim JM, Kim AR, Kim CJ, Kim YN. Effect of a kappa-opioid receptor agonist U50488H given at early reperfusion phase in isolated rat hearts. Korean J Anesthesiol 2008; 54: S29-S34.

2. Nayler WJ, Buckley DJ, Leong J. Calcium antagonists and the "stunned" myocardium. Cardioscience 1990; 1: 61-64. PMID:
2102798.

3. Liu X, Engelman RM, Wei Z, Bagchi D, Rousou JA, Nath D, et al. Attenuation of myocardial reperfusion injury by reducing intracellular calcium overloading with dihydropyridines. Biochem Pharmacol 1993; 45: 1333-1341. PMID:
8466553.


4. Nayler WG. Basic mechanisms involved in the protection of the ischemic myocardium. The role of calcium antagonists. Drugs 1991; 42: 21-27. PMID:
1718698.


5. Steenbergen C, Fralix TA, Murphy E. Role of increased cytosolic free calcium concentration in myocardial ischemic injury. Basic Res Cardiol 1993; 88: 456-470. PMID:
8117251.


6. Meissner A, Morgan JP. Contractile dysfunction and abnormal Ca
2+ modulation during postischemic reperfusion in rat heart. Am J Physiol 1995; 268: H100-H111. PMID:
7840255.


7. Wang QD, Pernow J, Sj├Čquist PO, Ryd├©n L. Pharmacological possibilities for protection against myocardial reperfusion injury. Cardiovasc Res 2002; 55: 25-37. PMID:
12062706.



8. Gourine AV, Pernow J, Poputnikov DM, Sj├Čquist PO. Calcium antagonist clevidipine reduces myocardial reperfusion injury by a mechanism related to bradykinin and nitric oxide. J Cardiovasc Pharmacol 2002; 40: 564-570. PMID:
12352318.


9. Utz J, Eckert R, Trautwein W. Inhibition of L-type calcium currents in guinea pig ventricular myocytes by the kappa-opioid agonist U50488H does not involve binding to opiate receptors. J Pharmacol Exp Ther 1995; 274: 627-633. PMID:
7543570.

10. Jang Y, Xi J, Wang H, Mueller RA, Norfleet EA, Xu Z. Postconditioning prevents reperfusion injury by activating delta-opioid receptors. Anesthesiology 2008; 108: 243-250. PMID:
18212569.


11. Chen Z, Li T, Zhang B. Morphine postconditioning protects against reperfusion injury in the isolated rat hearts. J Surg Res 2008; 145: 287-294. PMID:
18155248.


12. Guppy LJ, Littleton JM. Damaging effects of calcium paradox are reduced in isolated hearts from ethanol-dependent rats: paradoxic effects of dihydropyridine drugs. J Cardiovasc Pharmacol 1999; 34: 765-771. PMID:
10598118.


13. Tai KK, Bian CF, Wong TM. kappa-Opioid receptor stimulation increases intracellular free calcium in isolated rat ventricular myocytes. Life Sci 1992; 51: 909-913. PMID:
1355576.


14. Worthington MG, Opie LH. Effects of calcium channel agonism by Bay-K-8644 on ventricular fibrillation threshold of isolated heart. Cardiovasc Drugs Ther 1992; 6: 597-604. PMID:
1284030.


15. Laurent S, Girerd X, Tsoukaris-Kupfer D, Legrand M, Huchet-Brisac AM, Schmitt H, et al. Opposite central cardiovascular effects of nifedipine and BAY k 8644 in anesthetized rats. Hypertension 1987; 9: 132-138. PMID:
2434422.


16. Murphy JG, Marsh JD, Smith TW. The role of calcium in ischemic myocardial injury. Circulation 1987; 75: V15-V24. PMID:
3568336.

17. Murphy E, Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol Rev 2008; 88: 581-609. PMID:
18391174.


18. Shmist YA, Kamburg R, Ophir G, Kozak A, Shneyvays V, Appelbaum YJ, et al. N,N,N',N'-tetrakis(2-pyridylmethyl)-ethylenediamine improves myocardial protection against ischemia by modulation of intracellular Ca
2+ homeostasis. J Pharmacol Exp Ther 2005; 313: 1046-1057. PMID:
15681657.


19. Ferdinandy P, Appelbaum Y, Csonka C, Blasig IE, Tosaki A. Role of nitric oxide and TPEN, a potent metal chelator in ischemic and reperfused rat isolated hearts. Clin Exp Pharmacol Physiol 1998; 25: 496-502. PMID:
9673419.


20. Watts JA, Norris TA, London RE, Steenbergen C, Murphy E. Effects of diltiazem on lactate, ATP, and cytosolic free calcium levels in ischemic hearts. J Cardiovasc Pharmacol 1990; 15: 44-49. PMID:
1688981.


21. Bogaert MG. How do calcium channel blockers prevent cardiovascular events. Are they all alike? Drugs 1996; 52(Suppl 4): 3-7. PMID:
8913713.


22. Coetzee A, Conradie S. Calcium antagonist verapamil and reperfusion injury of the heart. J Cardiothorac Vasc Anesth 2007; 21: 337-343. PMID:
17544883.