 |
 |
|
|
Abstract
Anaphylaxis is an acute, potentially lethal, multisystem syndrome resulting from the sudden release of mast-cell- and basophile-derived mediators into the circulation. Common manifestations of anaphylactic reactions include urticaria, angioedema, nausea, vomiting, hypotension and cardiovascular collapse. Cardiovascular collapse is the first detected manifestation in up to 50% of cases in perioperative anaphylaxis, because patients are anesthetized and unable to report symptoms. A 25-year-old male presented with severe hypotension and erythema after intravenous atropine administration during general anesthesia. Postoperative laboratory findings demonstrated elevated serum tryptase and total immunoglobulin E. An intradermal test showed atropine sensitivity. Although atropine is used widely as a perioperative anticholinergic agent, it is a potential risk factor for a severe anaphylactic reaction. Therefore, prompt recognition and adequate therapeutic measures are necessary to avoid fatal consequences.
Anaphylaxis is a life-threatening hypersensitivity reaction. Perioperative anaphylaxis is rare and its incidence during general anesthesia is reported as 1 : 6000 to 1 : 20,000 [1,2,3,4]. Despite the rare incidence of anaphylaxis, mortality rates of 3-6% have been reported [5]. Common causative agents of anaphylaxis during general anesthesia include muscle relaxants, latex, antibiotics, and colloids [6]. Although atropine is used widely as an anticholinergic agent, associated anaphylaxis has been rarely reported in the literature [7,8]. Here, we report a case of successfully treated anaphylactic reaction triggered by atropine, the first such report in Korea, to assist establishment of guidelines for further management.
A 25-year-old male (height 167.7 cm, weight 52.2 kg) was admitted for surgical resection of a 5.0 ├Ś 1.8 cm right-cheek mass, suspected to be a lymphangioma. The patient had undergone a tonsillectomy under general anesthesia 8 years previously without any perioperative complication. The medical history indicated no drug or food allergies. Preoperative examinations of the patient were within normal limits. Therefore, anesthesia was applied according to the American Society of Anesthesiologists physical status classification 1.
The patient received no premedication prior to admission to the operating theater. The blood pressure upon arrival in the operating theater was 158/76 mmHg, with a heart rate of 80 beats/min (BPM), and with a peripheral oxygen saturation of 99% of room air. Anesthesia was induced using an injection of 50 ┬Ąg fentanyl, 150 mg propofol and 50 mg rocuronium. Endotracheal intubation was performed using a cuffed oral right-angle endotracheal tube. Anesthesia was maintained using oxygen, air and desflurane. As a prophylactic antibiotic, 1 g cefazoline was administered intravenously immediately after completion of induction.
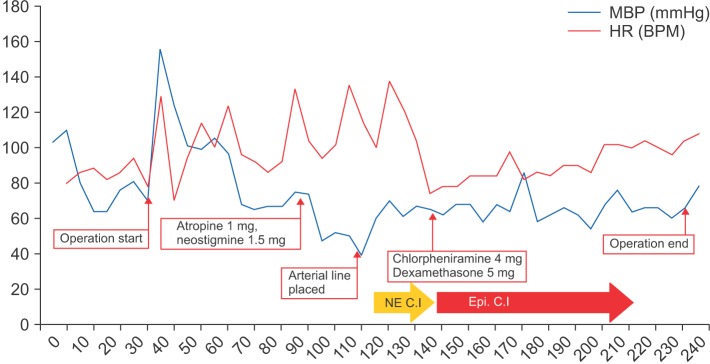
One hour after commencing surgery, the surgeon requested a reversal of the neuromuscular blockade to monitor patency of the facial nerve. Anesthesia was consequently maintained using desflurane alone. At this time, the blood pressure was 105/74 mmHg and the heart rate 92 BPM. We administered 1 mg atropine and 1.5 mg neostigmine intravenously to reverse neuromuscular blockade. After approximately 5 min, the blood pressure was decreased to 75/42 mmHg and the heart rate was 104 BPM. We administered 10 mg ephedrine intravenously. After 5 min, the blood pressure was 67/36 mmHg and the heart rate was 94 BPM. To manage persistent hypotension, 200 ┬Ąg phenylephrine were administered, followed by a rapid infusion of lactated Ringer's solution. However, the non-invasive blood pressure decreased further to 57/36 mmHg and the heart rate rose to 138 BPM. As we asked the surgeon, there was no drug administration at surgical field.
Invasive blood pressure monitoring was applied to the left dorsalis pedis artery and additional venous access was established. The arterial blood gas analysis showed all parameters to be within normal limits. Continuous infusion of norepinephrine at 0.03 ┬Ąg/kg/min was initiated. Consequently, the blood pressure was restored to 99/51 mmHg and surgery was resumed.
Because the patient was covered with a drape, clinical manifestation of anaphylaxis, including skin rash, could not be observed. Approximately 1 h after the hypotension event, a skin rash was detected over the entire body. There were no respiratory symptoms, including wheezing or increased airway pressure. The patient was suspected to be suffering from an anaphylactic reaction. A 10 ┬Ąg bolus of epinephrine was given and a continuous epinephrine infusion was initiated at 0.03 ┬Ąg/kg/min. The patient responded to the epinephrine and soon maintained hemodynamic stability; with a blood pressure of 110/52 mmHg and a heart rate of 78 BPM. Norepinephrine infusion was discontinued and epinephrine infusion was tapered to maintain the restored blood pressure. The patient was treated using 4 mg chlorpheniramine together with 5 mg dexamethasone to prevent delayed reactions and halt any further histamine release. After 1.5 h, the blood pressure was 110/46 mmHg without any inotropic or vasopressor support. The skin lesion was improved and no respiratory symptoms were detected throughout the completion of the surgery. The total operation time was 200 min and the anesthesia time 245 min. The total infused crystalloid, colloid, estimated blood loss and urine output were 1,300 ml, 500 ml, 50 ml, and 450 ml, respectively (Fig. 1).
The patient was transferred to the intensive care unit for close monitoring and was placed on ventilatory support. An hour after arrival, the patient was extubated uneventfully without any neurologic deficit. Blood tests were obtained for the tryptase level, which was elevated to 37.9 ┬Ąg/L (normal range: 0-11 ┬Ąg/L) and the total serum immunoglobulin E (IgE) level, which was increased to 675 Ku/L (normal range: negative). The patient was transferred to a general ward 1 day later and was discharged after 1 week without any complications. The consultant allergist recommended that allergen tests be conducted.
Skin-prick and intradermal tests were performed after 1 month to determine the cause of the anaphylaxis. The skinprick tests were negative for all agents, whereas the intradermal tests were positive for atropine (wheal of 4 ├Ś 4 mm for a stock concentration of 0.5 mg/ml; wheal of 4 ├Ś 4 mm with 1 : 5 dilution) (Table 1). The intradermal skin tests also demonstrated a positive result for rocuronium (wheal of 5 ├Ś 5 mm for a stock concentration of 10 mg/ml; wheal of 4 ├Ś 4 mm with 1 : 10 dilution). The patient was advised to completely avoid atropine and is currently receiving regular check-ups at the outpatient department of plastic surgery.
This case indicates the difficulties anesthesiologists encounter in diagnosing anaphylactic reactions after administration of an atypical antigen. Anaphylaxis is an acute, potentially lethal, multisystem syndrome resulting from the sudden release of mastcell- and basophil-derived mediators into the circulation [9,10].
Anaphylaxis is highly likely when one of three criteria is fulfilled. The first criterion includes the acute onset of illness with the involvement of the skin or mucosa, including hives, pruritus and angioedema, and either respiratory compromise or a systolic blood pressure < 90 mmHg (or symptomatic hypotension). A second criterion consists of two or more signs or symptoms occurring rapidly following exposure to a likely allergen. The third criterion is defined as a systolic blood pressure < 90 mmHg or symptomatic hypotension following exposure to a known allergen [11]. In the present case, hypotension, skin lesion and a positive skin test were found.
Perioperative anaphylaxis most commonly presents with cardiovascular, cutaneous and respiratory symptoms. Cardiovascular collapse is the first detected manifestation in up to 50% of cases [9]. Tachycardia is a classic cardiovascular sign of anaphylaxis, although bradycardia occasionally develops later in the reaction should the patient becomes hypoxemic or develop a heart block [12]. Bronchospasm may present as a sudden increase in the ventilatory pressure required to inflate the lungs, an increase in the end-tidal carbon dioxide or a decrease in the arterial oxygen saturation. A rapidly developing laryngeal edema may present as a difficulty with intubation or stridor postoperatively. In the current case, the heart rate increased to 130 BPM while the blood pressure fell abruptly. There were no respiratory signs.
Anaphylaxis during anesthesia may be particularly difficult to recognize for several reasons: Early or mild symptoms, including itching and shortness of breath, may go unrecognized because the patient cannot communicate. Secondly, cutaneous signs, including flushing, urticaria and angioedema, may be missed because the patient is draped for surgery. As a result of these factors, the reaction may be detected only upon dramatic respiratory and hemodynamic changes. In the present case, a skin lesion was not detected immediately on deterioration of the hemodynamic index.
Prompt initial treatment is essential in an anaphylactic episode, because even a few minutes' delay may lead to fatal complications, including hypoxic-ischemic encephalopathy and death. Although epinephrine is the first-line therapy for the emergency management of anaphylaxis, it is not always given promptly, even in hospitalized patients. As in the present case, anaphylaxis can be difficult to diagnose promptly during anesthesia; consequently, treatment using epinephrine may be delayed. In a retrospective study [13], 45% of the patients with anaphylaxis during anesthesia developed shock, circulatory instability or cardiac arrest, yet only 83% of these patients received epinephrine appropriately. It is important for the attending physician to consider any agent which may only rarely induce anaphylaxis, including atropine, as in the present case.
H1-antihistamines are not the drugs of choice in initial anaphylaxis treatment, because they do not relieve life-threatening respiratory symptoms or shock, although they do decrease urticaria and itching. Glucocorticoids are similarly not the drugs of choice in initial anaphylaxis treatment. However, they remain in use for anaphylaxis because they potentially prevent biphasic anaphylaxis [14].
The results of laboratory tests performed during anaphylaxis may be useful in some patients for subsequently confirming the diagnosis [14]. Serum tryptase is one of the mediators released by activated mast cells in immune-mediated anaphylactic reactions. The half-life of serum tryptase is 90 min, and an elevation in tryptase levels 1-2 h after anaphylaxis displays a positive correlation with anaphylaxis severity [15]. Serum or plasma to determine the tryptase level should be obtained ideally within the first 3 h after anaphylaxis. Elevated levels of active or total tryptase in the serum may be useful in distinguishing anaphylaxis from other conditions in the differential diagnosis, including vasovagal reactions, septic shock, seizures, myocardial shock, benign flushing, and carcinoid syndrome.
In the present case, the patient displayed anaphylaxis following intravenous administration of neuromuscular reversal agents. Taking into account the clinical features displayed during surgery and the time to reaction onset, we assumed that this case was a typical anaphylaxis. The elevated serum tryptase level demonstrated mast-cell mediator release. Elevated total IgE was further strong etiological evidence for anaphylaxis, which is mediated by IgE. However, it was difficult to identify the causative agent of anaphylaxis during surgery. We presumed that atropine or neostigmine would be the most likely candidate, because anaphylaxis usually occurs within a few minutes of administration. As far as we are aware, this is the first report of atropine-induced anaphylaxis in Korea.
Skin testing is an important element in the diagnosis of an IgE-mediated allergy. There are two commonly used methods of skin testing for IgE-mediated disorders: prick/puncture and intradermal tests. Intradermal tests are more reproducible than prick/puncture skin tests and are approximately 100- to 1000-fold more sensitive. However, false-positive reactions are more common, and this type of testing has a higher risk of inducing a systemic allergic reaction. After an anaphylactic episode, it is standard to delay skin tests for a minimum of 3-4 weeks [14]. An anaphylactic episode within the previous month is a contraindication for skin testing because it may yield false-negative results. Anaphylaxis can render the skin temporarily nonreactive. Full restoration of reactivity can take 2-4 weeks. When the intradermal skin test was conducted in the present case, both rocuronium and atropine displayed a positive reaction. Despite the possibility of the occurrence of delayed anaphylaxis, the positive finding of the intradermal test (1 : 10) for rocuronium is not a strong indicator of the reason for anaphylaxis, because of a high false-positive ratio. In the present case, it is less likely that delayed anaphylaxis resulted from rocuronium, because without a specific external stimuli to maintain a constant blood pressure, it suddenly fell, with a systolic blood pressure of 60 mmHg.
According to previous studies, 10% of anaphylaxis occurs as delayed anaphylaxis. Delayed anaphylaxis differs from anaphylaxis in that a series of symptoms occur gradually and to a lesser degree. The fact that the vital signs of the patient in the present case were kept steady before atropine injection, in response to which the systolic blood pressure fell abruptly to 60 mmHg while displaying no further symptoms of anaphylaxis, excludes the possibility of delayed anaphylaxis. Because anaphylaxis occurred immediately after intravenous injection of atropine and neostigmine, we could rule out induction agents such as rocuronium, rather suspecting atropine to be the allergen.
Ephedrine and phenylephrine rarely cause anaphylaxis. However, in the present case we cannot with certainty rule out ephedrine or phenylephrine as the causative agent of anaphylaxis. The abrupt decrease in the systolic blood pressure from 105 to 75 mmHg in the absence of any other external stimulus is rather difficult to explain should ephedrine or phenylephrine be the cause of anaphylaxis.
Atropine is a muscarinic acetylcholine receptor antagonist frequently used to treat symptomatic sinus bradycardia, AV block and the reversal of non-depolarizing neuromuscular blockade. Some cases of atropine-related anaphylaxis during an operation have been reported [7,8]. As neuromuscular monitoring becomes more common, atropine can be used as an adjuvant for reversal agents during surgery. Atropine, as a rare allergen, can trigger anaphylaxis during surgery. Anaphylaxis during surgery is a rare but life-threatening event, and anaphylaxis in response to the administration of rare, unusual agents, including atropine, as in the present case, makes the diagnosis even more difficult. Therefore, we recommend that anesthesiologists should be aware of the potential allergenicity of atropine.
In conclusion, anaphylactic reactions can occur in response to even to the most commonly used drugs during general anesthesia, including atropine. Anesthesiologists should always be aware of the possibility of anaphylactic reactions to all drugs used during anesthetic management. The initiation of aggressive therapeutic management is essential when anaphylaxis is strongly suspected, and tests to determine the specific drug(s) responsible for anaphylaxis should be conducted immediately after the operation to prevent further adverse events.
References
1. Chacko T, Ledford D. Peri-anesthetic anaphylaxis. Immunol Allergy Clin North Am 2007; 27: 213-230. viPMID: 17493499.


2. Lin RY, Anderson AS, Shah SN, Nurruzzaman F. Increasing anaphylaxis hospitalizations in the first 2 decades of life: New York State, 1990-2006. Ann Allergy Asthma Immunol 2008; 101: 387-393. PMID: 18939727.


3. Decker WW, Campbell RL, Manivannan V, Luke A, St Sauver JL, Weaver A, et al. The etiology and incidence of anaphylaxis in Rochester, Minnesota: a report from the Rochester Epidemiology Project. J Allergy Clin Immunol 2008; 122: 1161-1165. PMID: 18992928.



4. Poulos LM, Waters AM, Correll PK, Loblay RH, Marks GB. Trends in hospitalizations for anaphylaxis, angioedema, and urticaria in Australia, 1993-1994 to 2004-2005. J Allergy Clin Immunol 2007; 120: 878-884. PMID: 17931562.


5. Ebo DG, Fisher MM, Hagendorens MM, Bridts CH, Stevens WJ. Anaphylaxis during anaesthesia: diagnostic approach. Allergy 2007; 62: 471-487. PMID: 17441788.


6. Harper NJ, Dixon T, Dugue P, Edgar DM, Fay A, Gooi HC, et al. Suspected anaphylactic reactions associated with anaesthesia. Anaesthesia 2009; 64: 199-211. PMID: 19143700.



7. Coelho D, Fernandes T, Branga P, Malheiro D, Rodrigues J. Intraoperative anaphylaxis after intravenous atropine. Eur J Anaesthesiol 2007; 24: 289-290. PMID: 17087843.


8. Aguilera L, Martinez-Bourio R, Cid C, Arino JJ, Saez de Eguilaz JL, Arizaga A. Anaphylactic reaction after atropine. Anaesthesia 1988; 43: 955-957. PMID: 2975150.


9. Sheikh A, Hippisley-Cox J, Newton J, Fenty J. Trends in national incidence, lifetime prevalence and adrenaline prescribing for anaphylaxis in England. J R Soc Med 2008; 101: 139-143. PMID: 18344471.



10. Liew WK, Williamson E, Tang ML. Anaphylaxis fatalities and admissions in Australia. J Allergy Clin Immunol 2009; 123: 434-442. PMID: 19117599.


11. Sampson HA, Mu├▒oz-Furlong A, Campbell RL, Adkinson NF Jr, Bock SA, Branum A, et al. Second symposium on the definition and management of anaphylaxis: summary report--Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol 2006; 117: 391-397. PMID: 16461139.


12. Gaeta TJ, Clark S, Pelletier AJ, Camargo CA. National study of US emergency department visits for acute allergic reactions, 1993 to 2004. Ann Allergy Asthma Immunol 2007; 98: 360-365. PMID: 17458433.


13. Garvey LH, Belhage B, Kroigaard M, Husum B, Malling HJ, Mosbech H. Treatment with epinephrine (adrenalin) in suspected anaphylaxis during anesthesia in Denmark. Anesthesiology 2011; 115: 111-116. PMID: 21572319.


14. Simons FE, Ardusso LR, Bilo MB, Dimov V, Ebisawa M, El-Gamal YM, et al. 2012 Update: World Allergy Organization (WAO) guidelines for the assessment and management of anaphylaxis. Curr Opin Allergy Clin Immunol 2012; 12: 389-399. PMID: 22744267.


15. Sala-Cunill A, Cardona V, Labrador-Horrillo M, Luengo O, Esteoso O, Garriga T, et al. Usefulness and limmitations of sequential serum tryptase for the diagnosis of anaphylaxis in 102 patients. Int Arch Allergy Immunol 2013; 160: 192-199. PMID: 23018683.


Fig.┬Ā1
Intraoperative Vital Signs. MBP: median blood pressure, HR: heart rate, NE C.I: norepinephrine continuous infusion, Epi. C.I: epinephrine continuous infusion.
Table┬Ā1
Results of Skin Test
- TOOLS








