|
 |
|
|
Abstract
Background
An important concern of intra-operative infusion of remifentanil is the possible development of acute opioid tolerance, which manifests as an increased postoperative analgesia requirement. We have examined the effect of the timing of intra operative morphine administration on the need for morphine consumption for pain control during the first 24 hours after operation.
Methods
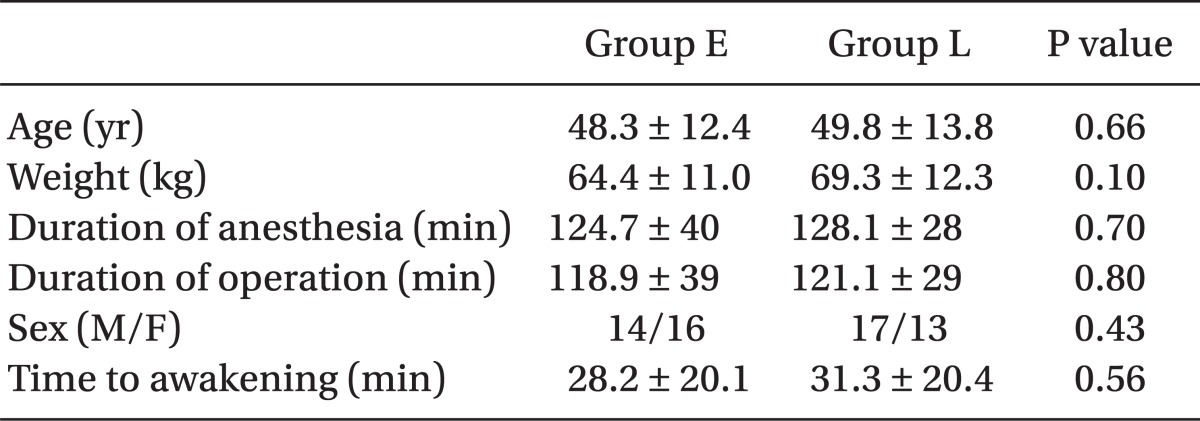
Sixty adult patients scheduled for elective open unilateral nephrolithotomy surgery were recruited for this prospective randomized double-blind study. Anesthesia was induced with 0.03 mg/kg midazolam, 1 ┬Ąg/kg remifentanil, and 1.5-2 mg/kg propofol. Anesthesia was maintained with 100 ┬Ąg/kg/min propofol, and 0.25 ┬Ąg/kg/min remifentanil. Both groups received 0.1 mg/kg morphine intravenously at 2 different times; in the first group (group E) immediately after intubation and in the second group (group L) 20-30 min before the anticipated end of operation.
Results
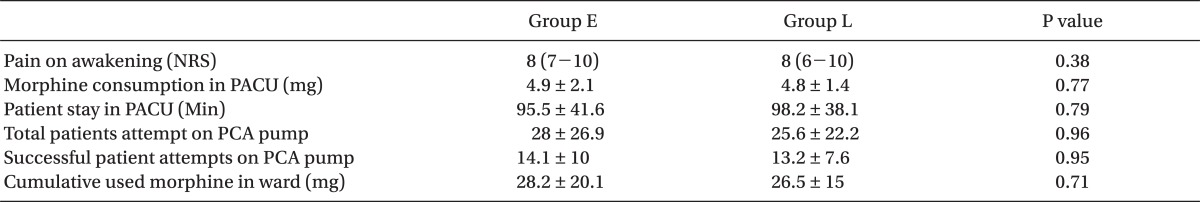
There was no difference in pain scores at awakening, the amount of morphine given to the 2 groups for pain control, or the time to discharge from PACU between the 2 groups. The pain scores at admission to ward and at every 4 hours thereafter, until 24 hours, were not significantly different between the 2 groups. The cumulative amount of the first 24 hours morphine consumption in the ward in E group was 28.2 ┬▒ 20.1 mg and 26.5 ┬▒ 15 mg in L group, respectively (P = 0.71).
Conclusions
Early intra-operative administration of morphine compared to that of morphine in the end of surgery did not affect postoperative morphine consumption and pain scores during the first 24 hours after surgery for open nephrolithotomy. Newer pharmacologic interventions for prevention of acute tolerance of opioids seems rational (Clinical trial registration No. ACTRN: 12609000570280).
Potent opioids with short duration of action are widely used as analgesic agents in anesthesia. Intra-operative infusion of remifentanil hydrochloride with propofol is an established technique that is commonly used to meet anesthesia needs. The ultra-short duration of action of remifentanil is independent of dose, allowing infusion of large doses throughout surgery with little risk of postoperative delayed awakening or respiratory depression [1,2]. However, this characteristic may be a disadvantage at the end of anesthesia as the rapid cessation of the effect of remifentanil can result in early and severe postoperative pain if the analgesic regimen has not been started prior to the end of surgery [3,4].
Another important concern with intraoperative infusion of remifentanil is the possible development of acute opioid tolerance and/or hyperalgesia, manifesting as an increased requirement for postoperative analgesic [5-8]. There is evidence that a high-dose infusion of a short-acting opioid during painful surgery may induce tolerance more rapidly than would be expected with a long-acting drug [9-14]. Profound tolerance has been demonstrated after only 60-90 min of remifentanil infusion in volunteers, [14] but the clinical consequences of acute tolerance to remifentanil have not been evaluated to date.
Various methods have been evaluated to attenuate the development of remifentanil induced hyperalgesia; the majority of these methods have focused on the N-methyl-D-aspartate antagonist, ketamine, with little if any benefit [15-17] Rotational regimens or combination of opioids have been proposed to decrease or prevent opioid-induced hyperalgesia [18]. In this study, we measured the total amount of morphine consumed by patients for postoperative pain control and pain scores during the first 24 hours after surgery.
After approval by the Ethics Committee of Shiraz University of Medical Sciences a written informed consent was obtained from each participating patient, all of which were 60 adults aged 40-70 years old, with American Society of Anesthesiologists physical status I or II scheduled to undergo elective open unilateral nephrolithatomy were recruited for this prospective randomized double-blind study. Patients were excluded preoperatively if they had a history of acute or chronic kidney injury, drug abuse, received any analgesic medications during the previous 48 hours or were unable to use a patient-controlled analgesia device. The patients were instructed to explain their pain according to a numerical rating scale (NRS) from zero (no pain) to 10 (worst imaginable pain). Randomization of the subjects using a computer generated random numbers was performed on the day of surgery.
All patients received premedication with 5-10 mg diazepam the night before their operation. Electrocardiography, pulse oximetry, capnography, and non-invasive blood pressure monitoring was used in all patients. After preoxygenation, anesthesia was induced with 0.03 mg/kg midazolam, 1 ┬Ąg/kg remifentanil, and 1.5-2 mg/kg propofol. Muscle relaxation was provided by 0.5 mg/kg atracurium and endotracheal intubation was done after 3 min of bag and mask ventilation. Mechanical ventilation was started to maintain normocarbia. Anesthesia was maintained with 100 ┬Ąg/kg/min propofol, 0.25 ┬Ąg/kg/min remifentanil, and a mixture of O2 and N2O in 50 : 50 ratios. Both groups received 0.1 mg/kg morphine intravenously diluted in normal saline to a concentration of 1 mg/ml at 2 different times; in the first group (group E) immediately after intubation and in the second group (group L) 20-30 min before the anticipated end of operation. The remifentanil infusion was adapted by the anesthetist in steps of 0.05 ┬Ąg/kg/min according to variations of heart rate and arterial systolic pressure. Heart rate and systolic blood pressure were kept within 20% of pre-induction values. The patient's bladder was catheterized according to routine practice.
Intravenous fluids were infused according to the anesthetist's discretion. After skin closure; end of surgery, propofol, and remifentanil administration were discontinued and residual neuromuscular blockade was antagonized with intravenous neostigmine and atropine. The trachea was extubated when patients where responsive to verbal commands, end-tidal carbon dioxide partial pressure was acceptable, and tidal volume was more than 5 mg/kg. Patients were transferred to the post anesthesia care unit (PACU) within 5 min after tracheal extubation. After awakening in PACU (T1), the pain score was recorded according to numerical rating scale by a nurse, who was blinded to the patient study assignments. If the NRS was more than 3, morphine was given intravenously, in increments of 1 mg, every 10 min until the pain score decreased to 3 or less. The total dose of morphine needed was recorded. Next, a patient-control analgesia pump (Microject, Sorenson Medical and West Jordan, UT, USA) was started. The morphine solution was prepared in 20 ml boluses with a concentration of 1 mg/ml. The interval lockout time was 10 min and bolus dose was set to 2 mg without a background infusion. The maximum permissible dose was 20 mg.
Discharge from the PACU was with recommendation of the nurse and at the discretion of the anesthetist (T2). Each patient's pain score at the arrival to ward (T3) and at every 4 hours thereafter, up to 24 hours were recorded by nurses blinded to treatment. No other analgesics were administrated during the 24 hours observation period. The recorded NRS in each time scale, the total number of successful and unsuccessful attempts of patients to activate the PCA pump, and total morphine consumption were recorded and compared between 2 groups with appropriated statistical tests.
The sample size was chosen on the basis of similar studies with a power of 90% with an ╬▒ level of 0.05 and a minimal difference to be detected in a post-operative morphine consumption of 30%. Patient characteristics and clinical variables were compared using X2-test or t-test. Repeated measures analysis of variance was used for comparison of morphine consumption. The Mann-Whitney rank sum test was used for comparison of NRS pain scores. Data analysis was performed using SPSS software version 13 (Chicago, Il, USA).
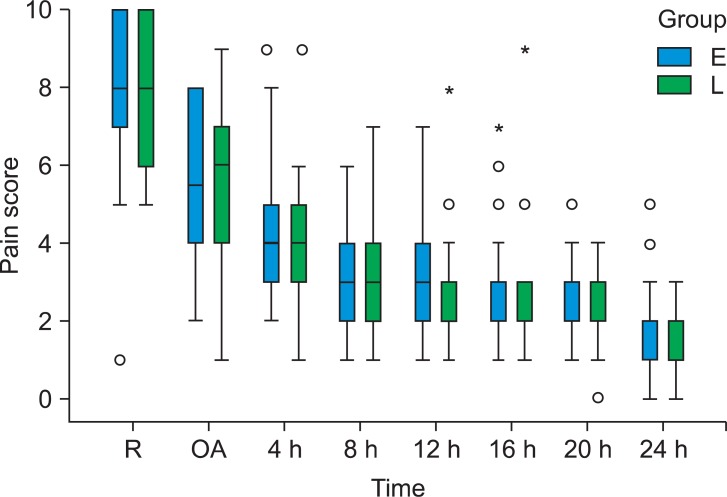
All 60 patients completed the study. Both groups were similar with respect to age, weight, gender, duration of anesthesia, and surgery and hemodynamic variables during the operation (Table 1). In addition, there was also no difference in pain scores at awakening, morphine given to the two groups for pain control and time to discharge from PACU between the 2 groups (Table 2). There was no difference in pain scores (NRS) at admission to ward and at every 4 hours thereafter until 24 hours between the 2 groups. Median and 25-75% interquartile numeric rating scale pain scores of the patients in the recovery room, on arrival to ward, and at every 4 hours thereafter are shown in Fig. 1.
The cumulative dose of morphine used by the patients during the first post-op day was similar, as well. The cumulative first 24 hours morphine consumption in the ward in E group was 28.2 ┬▒ 20.1 mg and 26.5 ┬▒ 15 mg in L group (P = 0.71). No patient experienced a serious adverse effect. The total number of the patient attempts to activate the PCA pump and total number of successful drug delivery to the patients were similar in both groups (P = 0.96) (Table 2).
In this study, the time of morphine administration during the operation did not have any significant effect on the amount of morphine, post-operative, for pain control during the first 24 hours. This is not in agreement with the untested clinical practice that uses a bolus of morphine prior to initiating remifentanil by infusion. However, this finding, is in agreement with 2 recent investigations in which fentanyl or morphine pretreatment in orthopedic surgery was used to reduce acute tolerance to remifentanil [19,20].
Acute tolerance is reported by administration of many opioids including morphine, sufentanil, and alfentanil [9,10,12,13,21,22]; the intensity of tolerance has been reported by some investigators to be unrelated to the potency of the opioid used [13]. Although opioids with shorter duration of action show a faster development of tolerance, it is likely that the use of a different opioid, other than morphine, is not a good choice for preventing acute tolerance.
We could not comment on the development of remifentanil-induced hyperalgesia because we considered it unethical to include a group with no morphine consumption perioperatively. However, based on previous studies [5-8], it is not unusual to see this phenomenon when low doses of remifentanil are used to treat pain for surgical operations. Even if our patients were susceptible, based on current study, we couldn't find any relation between the timing of morphine administration and remifentanil-induced hyperalgesia.
Some investigators recommend that development of remifentanil-induced hyperalgesia is related to duration and dose of used remifentanil rather than time of morphine administration [5,19,23]. This is in agreement with our findings as the duration of anesthesia and surgery was very similar in both groups in our study. We titrated the dose of remifentanil according to hemodynamic variables in our study, and we didn't saw any significant difference on analgesic needs between the 2 groups; remifentanil consumption was similar during the operation. However, this study was not powered to detect this difference in the analgesic requirements.
Mu├▒oz et al. [3] looked at the effect of timing of morphine administration on early post-operative pain score and recovery condition of patients after laparoscopic cholecystectomy with remifentanil based anesthesia. These researchers concurred with our results, in that they did not find any significant difference in patient pain scores in the recovery period; however, they reported lower pain scores when 150 ┬Ąg/kg morphine had been given more than 40 min before the end of surgery. This result was attributed by the investigators to a better matching between the time of morphine administration and its peak analgesia effect at the moment of arrival at the PACU rather than pre-emptive effects of morphine [3].
In conclusion, our study shows that earlier use of morphine during remifentanil-based anesthesia causes no less morphine consumption during the first 24 hours after surgery for open nephrolithatomy. We suggest future studies on analgesic requirements after remifentanil-based anesthesia that focus on the effect of the total dose and duration of remifentanil infusion on the development of acute tolerance. New pharmacologic interventions for prevention of this undesirable side effect seem rational as well.
Acknowledgments
The authors would like to thank Leila Ghazanfari for typing the manuscript and Clinical Research Development Center in Nemazee Hospital of statistical analysis.
References
1. Crawford MW, Hickey C, Zaarour C, Howard A, Naser B. Development of acute opioid tolerance during infusion of remifentanil for pediatric scoliosis surgery. Anesth Analg 2006; 102: 1662-1667. PMID: 16717305.


2. Thompson JP, Rowbotham DJ. Remifentanil--an opioid for the 21st century. Br J Anaesth 1996; 76: 341-343. PMID: 8785129.



3. Mu├▒oz HR, Guerrero ME, Brandes V, Cort├Łnez LI. Effect of timing of morphine administration during remifentanil-based anaesthesia on early recovery from anaesthesia and postoperative pain. Br J Anaesth 2002; 88: 814-818. PMID: 12173199.



4. Albrecht S, Schuttler J, Yarmush J. Postoperative pain management after intraoperative remifentanil. Anesth Analg 1999; 89(4 Suppl): S40-S45. PMID: 10511077.

5. Guignard B, Bossard AE, Coste C, Sessler DI, Lebrault C, Alfonsi P, et al. Acute opioid tolerance: Intraoperative remifentanil increases postoperative pain and morphine requirement. Anesthesiology 2000; 93: 409-417. PMID: 10910490.


6. Jo HR, Chae YK, Kim YH, Chai HS, Lee WK, Choi SS, et al. Remifentanil-Induced pronociceptive effect and its prevention with pregabalin. Korean J Anesthesiol 2011; 60: 198-204. PMID: 21490822.



7. Joly V, Richebe P, Guingnard B, Fletcher D, Maurette P, Sessler DI, et al. Remifentanil-induced postoperative hyperalgesia and its prevention with small-dose ketamine. Anesthesiology 2005; 103: 147-155. PMID: 15983467.


8. Angst MS, Koppert W, Pahl I, Clark DJ, Schmelz M. Short-term infusion of the mu-opioid agonist remifentanil in humans causes hyperalgesia during withdrawal. Pain 2003; 106: 49-57. PMID: 14581110.


9. Kissin I, Brown PT, Robinson CA, Bradly EL Jr. Acute tolerance in morphine analgesia: continuous infusion and single injection in rats. Anesthesiology 1991; 74: 166-171. PMID: 1986642.


10. Kissin I, Lee SS, Arthur GR, Bradley EL Jr. Time coarse characteristics of acute tolerance development to continuously infused alfentanil in rats. Anesth Analg 1996; 83: 600-605. PMID: 8780289.


11. Larcher A, Laulin JP, Celerier E, Le Moal M, Simonnet G. Acute tolerance associated with a single opiate administration: Involvement of N-methyl-D-aspartate-dependent pain facilitatory systems. Neuroscience 1998; 84: 583-589. PMID: 9539228.


12. Cox BM, Ginsburg M, Osman OH. Acute tolerance to narcotic analgesic drugs in rats. Br J Pharmacol Chemother 1968; 33: 245-256. PMID: 5664145.



13. Kissin I, Brown PT, Bradly EL Jr. Magnitude of acute tolerance to opioids is not related to their potency. Anesthesiology 1991; 75: 813-816. PMID: 1659255.


14. Vinik HR, Kissin I. Rapid development of tolerance to analgesia during remifentanil infusion in humans. Anesth Analg 1998; 86: 1307-1311. PMID: 9620525.


15. Van Elstraete AC, Lebrun T, Sandefo I, Polin B. Ketamine does not decrease postopearative pain after remifentanil-based anesthesia for tonsillectomy in adults. Acta Anaesthesiol Scand 2004; 48: 756-760. PMID: 15196109.


16. Ganne O, Abisseror M, Menault P, Malhi├©re S, Chambost V, Charpiat B, et al. Low-dose ketamine failed to spare morphine after a remifentanil-based anesthesia for ear, nose and throat surgery. Eur J Anaesthesiol 2005; 22: 426-430. PMID: 15991504.


17. Batra YK, Shamsah M, Al-Khasti MJ, Rawdhan HJ, Al-Qattan AR, Belani KG. Intraoperative small-dose ketamine does not reduce pain or analgesic consumption during perioperative opioid analgesia in children after tonsillectomy. Int J Clin Pharmacol Ther 2007; 45: 155-160. PMID: 17416110.


18. Koppert W. Opioid-induced hyperalgesia. Pathophysiology and clinical relevance. Anaesthesist 2004; 53: 455-466. PMID: 15034638.


19. McDonnell C, Zaarour C, Hull R, Thalayasingam P, Pehora C, Ahier J, et al. Pre-treatment with morphine does not prevent the development of remifentanil-induced hyperalgesia. Can J Anaesth 2008; 55: 813-818. PMID: 19050084.


20. Lenz H, Raeder J, Hoymork SC. Administration of fentanyl before remifentanil-based anaesthesia has no influence on post-operative pain or analgesic consumption. Acta Anaesthesiol Scand 2008; 52: 149-154. PMID: 17996006.


21. Ling GS, Paul D, Simantov R, Pasternak GW. Differential development of acute tolerance to analgesia, respiratory depression, gastrointestinal transit and hormone release in a morphine infusion model. Life Sci 1989; 45: 1627-1636. PMID: 2555641.


22. Ho ST, Wang JJ, Liaw WJ, Lee HK, Lee SC. Surgical pain attenuates acute morphine tolerance in rats. Br J Anaesth 1999; 82: 112-116. PMID: 10325847.



23. Rauf K, Vohra A, Fernandez-Jimenez P, O'Keeffe N, Forrest M. Remifentanil infusion in association with fentanyl-propofol anaesthesia in patients undergoing cardiac surgery: effects of morphine requirement and postoperative analgesia. Br J Anaesth 2005; 95: 611-615. PMID: 16155034.



Fig.┬Ā1
Box and whisker plots showing median and 25-75% interquartile, numeric rating scale pain scores of the patients in the recovery room (R), on arrival to ward (OA) and every 4 hours thereafter (Group E: Early morphine, Group L: Late morphine). Circles represent points that are > 1.5 times the interquartile range. Asterisks represent points that are > 3 times the interquartile range. *Than three times the interquartile range.
- TOOLS