 |
 |
|
|
Abstract
Background
A microemulsion propofol causes a high incidence of pain during intravenous injection. In this study, we investigated the effect of ramosetron on pain induced by microemulsion propofol injection.
Methods
After prospective power analysis and institutional review board approval, a total of 200 ASA I and II patients undergoing general anesthesia were divided into 4 groups. They received one of the following intravenously after tourniquet application on the forearm 1 min before induction of anesthesia using microemulsion propofol; normal saline (Group N, n = 50), lidocaine 20 mg (Group L, n = 50), ramosetron 0.3 mg (Group R, n = 50) and lidocaine 20 mg plus ramosetron 0.3 mg (Group LR, n = 50) diluted into a 5 ml solution. The occlusion was released after 30 seconds and microemulsion propofol was injected over 10-15 seconds. The patients were observed and asked immediately if they had pain in the arm, and their responses were assessed.
Lipid emulsion propofol has been widely used for the induction and maintenance of general anesthesia due to its faster onset time and shorter action duration. Hyperlipidemia, pulmonary fat embolism, pancreatitis, propofol infusion syndrome, and drug contamination have been reported to be complications associated with lipid solvents [1]. In particular, injection pain has been reported to have a 68-90% prevalence, and has been regarded as a serious problemit ranks seventh among the top 33 clinical problems of anesthesia [2-5]. To resolve the complications associated with lipid solvents, development of altered lipid emulsion or non-emulsion formulations has been tried [1,6,7]. Microemulsion propofol (Aquafol®, Daewon Pharmaceutical Co., Ltd., Seoul, South Korea), which was recently developed and has since been started to be used, has the advantage of minimizing the complications associated with lipid solvent of the existing propofol. But it has the disadvantage of causing more frequent and severer pain compared to existing propofol in terms of injection pain [8]. Injection pain is not only a cause of pain experienced by patients but also a subject of interest to anesthesiologists who use propofol. Various methods have been used to resolve propofol injection pain, but no study has reported a method that removes the pain completely [3, 9-11]. Recently, 5-HT3 receptor antagonists, which are used as antiemetics, were found to have characteristics of local anesthetics were effective in the prevention of injection pain caused by propofol [10,12,13].
It was hypothesized by these authors that ramosetron, a type of 5-HT3 receptor antagonist, reduces injection pain caused by microemulsion propofol. Accordingly, we conducted a study to investigate the effect of ramosetron on the injection pain caused by microemulsion propofol in patients who had been scheduled to undergo surgery under general anesthesia.
Two hundred healthy patients aged 20-60 years who were scheduled to undergo surgery under general anesthesia and who belonged to physical status classification I or II of the American Society of Anesthesiologists (ASA) were selected as subjects. Excluded from the study were patients: who had an experience of hypersensitivity to local anesthetics and antiemetics; who had asthma, neurological disorders, or took analgesics or sedatives within 24 hours before surgery; and who had weak or thin blood vessels into which drug is injected were. The study was approved by our IRB and was conducted after obtaining informed consent from each patient. No significant baseline differences in age, gender, height, and weight were found between groups (Table 1).
No patients received drugs for hypnosis and sedation. An 18-gauge catheter was catheterized in the hand vein for the injection pathway before arrival at the operation room. Upon arrival at the operation room, blood pressure, SpO2, and ECG were monitored. Patients were randomly assigned to one of the four groups using a random number table. One minute before microemulsion propofol injection, a tourniquet was installed. Normal saline (group N, n = 50), lidocaine 20 mg (group L, n = 50), ramosetron 0.3 mg (group R, n = 50), or ramosetron 0.3 mg and lidocaine 20 mg (group LR, n = 50) were diluted with normal saline and injected 5 ml volume. Thirty seconds after drug injection, the tourniquet was removed, and one-fourth of a microemulsion propofol dose (2 mg/kg), an anesthesia induction dose, was injected. All patients were asked if they felt arm pain or discomfort during drug injection. Pain was divided into four stages, and the stage was recorded. The pain severity classification was as follows: 0 (no pain), 1 (mild pain; mild movement or oral/facial response during injection), 2 (intermediate pain; clear movement or oral/facial response during injection), and 3 (severe pain; complaint of pain and withdrawal response of the upper extremities). After pain assessment, the remaining dose was injected to induce anesthesia.
Based on literature references [14], intermediate or severe injection pain due to microemulsion propofol was assumed to have an 82% frequency. Forty-percent pain reduction in the intermediate or severe level after preventive drug injection was considered clinically significant. Fifty patients were calculated as the minimum size for each group assuming an α-value of 0.05 and a power value of 80%. SPSS (Windows ver. 17.0, SPSS Inc., Chicago, IL) was used for statistical analysis. All measured values are presented as mean ± standard deviation, or numbers (%). For age, height, and weight, one-way ANOVA test was used, and if a significant difference was found, a Bonferroni Post-Hoc test was used. For gender, ASA class, and injection pain frequency, χ2 tests were used for analysis. P values < 0.05 for age, height, and weight, and P values < 0.008 for gender, ASA class, and injection pain frequency were considered statistically significant.
A total of 200 patients were analyzed - no patient was withdrawn due to pain during the study. After injecting microemulsion propofol (one-fourth of the anesthesia induction dose), the overall incidence of injection pain was 96, 76, 60, and 38% in groups N, L, R, and LR, respectively. The overall incidence of pain was significantly lower in groups L, R, and LR than in group N. No significant difference was found between groups L and R (Table 2, P < 0.008). A more significant reduction of injection pain occurrence was seen in group LR compared to group L (Table 2, P < 0.008). The incidence of moderate to severe pain was significantly lower in groups LR (n = 2, 4%), R (n = 9, 18%), and L (n = 20, 40%) than in group N (n = 42, 84%), and significantly lower in group LR than in group L (Table 2, P < 0.008).
In this study, the incidence of injection pain was 96% in the placebo group. The incidence in the groups pretreated with ramosetron 0.3 mg or combination with ramosetron and lidocaine 20 mg were 60% and 38%, respectively. These results show effective reduce in injection pain. Pretreatment with ramosetron alone or with combined pretreatment of ramosetron and lidocaine also prevented pain effectively for moderate to severe pain.
Injection pain associated with propofol charactically occurs immediately or later, after drug injection, and has a delayed response of 10-20 seconds [15]. Microemulsion propofol is safer than existing-propofol fromulations due to the removel of serious adverse events of propofol in the lipid emulsion state. It has the disadvantages, however, of severe pain and difficulty when it is used alone [8,14]. Jung et al. [14] reported that the incidence of moderate to severe pain after microemulsion propofol injection was 81.9%, which was higher than the injection of propofol in the lipid emulsion state (29.2%). Although the cause of higher incidence has not yet been identified, one possibility, which has drawn much attention, is that the free propofol concentration is sevenfold higher in microemulsion propofol than in lipid emulsion propofol [8,16]. In additon, it has been reported that injection pain may occur because free propofol directly stmulates the free nerve ending and A-delta myelinated nociceptors [8,14,17]. Various methods have been used for the reduction of pain caused by nociceptive receptor stimulation, but no method that resolves pain completely has been found to date [9,10]. Mono-treatment or combination pretreatment of local anesthetics, narcotic analgesics, low ketamine, and ondansetron have been widely used and they significantly reduce the incidence and severity of injection pain [4,10,18-21].
As lidocaine has both a local anesthetic effect and a kinincascade-stabilizing effect, it can be used for injection pain prevention [22]. Venous flow inhibition of the upper extremities using a tourniquet can be effectivly used as a model for studying peripheral reactions to drugs without whole-body effects [23]. Picard and Tramer [24] analyzed 56 research studies, reporting that the best way to prevent injection pain is to inhibit blood flow using a tourniquet, administer lidocaine 40 mg for pretreatment, remove the tourniquet 30-120 seconds after the pretreatment, and inject propofol. Johnson et al. [3] reported that lidocaine 40 mg was more effective than lidocaine 20 mg in injection pain prevention. In addition, King et al. [25] reported that lidocaine 20 mg (32%) was more effective than normal saline (73%) in the prevention of injection pain caused by propofol.
This study was conducted by setting the tourniquet compression time at 30 seconds, according to the method suggested by Picard and Tramer [24]. As lidocaine may cause other complications, including cardiovascular and neurological toxicity, lidocaine 20 mg, which had been shown to be effective in a study conducted by King et al., was used in this study. 20 mg lidocaine injection was found to be effective in reducing injection pain caused by microemulsion propofol, but the overall incidence of injection pain was higher. This result is likely to be attributable to the fact that the lidocaine dose that was used in this study was lower than that suggested by Picard and Tramer.
5-HT3 receptor antagonists have been widely used as antiemetics. Ye et al. [13] reported that ondansetron, a 5-HT3 receptor antagonist, blocked the sodium channel in an animal study, and had a 15-fold-higher local anesthetic efficacy than lidocaine. In addition, as 5-HT3 receptor antagonists act as agonists by combining with the µ receptor, and as peripheral 5-HT3 receptors are involved in the nociceptive pathway, the 5-HT3 receptor antagonist results in an analgesic effect [26]. Therefore, 5-HT3 receptor antagonists can be used for the prevention of injection pain caused by microemulsion propofol. Ambesh et al. [10] conducted a clinical study on 80 patients and reported that propofol injection pain decreased by 25% in the group that had been pretreated with ondansetron 4 mg compared to the group with normal saline (55%). Although no study on the local anesthetic effect of ramosetron, a recently developed 5-HT3 receptor antagonist, has been conducted, and its role as an agonist in combination with the µ receptor has been demonstrated, this study was conducted with the assumption that ramosetron acts as ondansetron does. Ramosetron has been used during anesthesia induction or before the end of surgery to prevent nausea and vomiting after surgery or anticancer treatment [27,28]. In this study, ramosetron 0.3 mg, a single injection dose in adults with postoperative nausea and vomiting, was used. Ramosetron alone or combined with lidocaine reduce injection pain caused by propofol. The injection pain was shown to be higher in this study than in the study conducted by Ambesh et al. [10], where ondansetron 4 mg was used for pretreatment. This difference is likely to be attributable to the fact that the microemulsion propofol that was used in this study caused more severe pain than the propofol that was used in previous studies, and that the tourniquet compression time to obtain the same effect as that of local anesthetics was short.
In conclusion, the injection of ramosetron alone or combined with lidocaine significantly reduced the injection pain due to microemulsion propofol. A study on the effect of microemulsion propofol injection pain according to lidocaine dose change and tourniquet compression time is required.
References
1. Devlin JW, Lau AK, Tanios MA. Propofol-associated hypertriglyceridemia and pancreatitis in the intensive care unit: an analysis of frequency and risk factors. Pharmacotherapy 2005; 25: 1348-1352. PMID: 16185179.


2. Macario A, Weinger M, Truong P, Lee M. Which clinical anesthesia outcomes are both common and important to avoid? The perspective of a panel of expert anesthesiologists. Anesth Analg 1999; 88: 1085-1091. PMID: 10320175.


3. Johnson RA, Harper NJ, Chadwick S, Vohra A. Pain on injection of propofol. Methods of alleviation. Anaesthesia 1990; 45: 439-442. PMID: 2200299.


4. King SY, Davis FM, Wells JE, Murchison DJ, Pryor PJ. Lidocaine for the prevention of pain due to injection of propofol. Anesth Analg 1992; 74: 246-249. PMID: 1731545.


5. Smith I, White PF, Nathanson M, Gouldson R. Propofol. An update on its clinical use. Anesthesiology 1994; 81: 1005-1043. PMID: 7943815.


6. Baker MT, Naguib M. Propofol: the challenges of formulation. Anesthesiology 2005; 103: 860-876. PMID: 16192780.


7. Kim KM, Choi BM, Park SW, Lee SH, Christensen LV, Zhou J, et al. Pharmacokinetics and pharmacodynamics of propofol microemulsion and lipid emulsion after an intravenous bolus and variable rate infusion. Anesthesiology 2007; 106: 924-934. PMID: 17457123.


8. Sim JY, Lee SH, Park DY, Jung JA, Ki KH, Lee DH, et al. Pain on injection with microemulsion propofol. Br J Clin Pharmacol 2009; 67: 316-325. PMID: 19220277.



9. Kobayashi Y, Naganuma R, Seki S, Aketa K, Ichimiya T, Namiki A. Reduction of pain on injection of propofol: a comparison of fentanyl with lidocaine. Masui 1998; 47: 963-967. PMID: 9753961.

10. Ambesh SP, Dubey PK, Sinha PK. Ondansetron pretreatment to alleviate pain on propofol injection: a randomized, controlled, double-blinded study. Anesth Analg 1999; 89: 197-199. PMID: 10389803.

11. Gajraj NM, Nathanson MH. Preventing pain during injection of propofol: the optimal dose of lidocaine. J Clin Anesth 1996; 8: 575-577. PMID: 8910180.


12. Deegan R. Ondansetron: pharmacology of a specific 5HT3-receptor antagonist. Am J Med Sci 1992; 304: 373-378. PMID: 1456277.


13. Ye JH, Mui WC, Ren J, Hunt TE, Wu WH, Zbuzek VK. Ondansetron exhibits the properties of a local anesthetic. Anesth Analg 1997; 85: 1116-1121. PMID: 9356111.


14. Jung JA, Choi BM, Cho SH, Choe SM, Ghim JL, Lee HM, et al. Effectiveness, safety, and pharmacokinetic and pharmacodynamic characteristics of microemulsion propofol in patients undergoing elective surgery under total intravenous anaesthesia. Br J Anaesth 2010; 104: 563-576. PMID: 20299348.



15. Briggs LP, Clarke RS, Dundee JW, Moore J, Bahar M, Wright PJ. Use of di-isopropyl phenol as main agent for short procedures. Br J Anaesth 1981; 53: 1197-1202. PMID: 6976790.



16. Lee EH, Lee SH, Park DY, Ki KH, Lee EK, Lee DH, et al. Physicochemical properties, pharmacokinetics, and pharmacodynamics of a reformulated microemulsion propofol in rats. Anesthesiology 2008; 109: 436-447. PMID: 18719441.


17. Doenicke AW, Roizen MF, Rau J, Kellermann W, Babl J. Reducing pain during propofol injection: the role of the solvent. Anesth Analg 1996; 82: 472-474. PMID: 8623945.

18. Zahedi H, Nikooseresht M, Seifrabie M. Prevention of propofol injection pain with small-dose ketamine. Middle East J Anesthesiol 2009; 20: 401-404. PMID: 19950734.

19. Fujii Y, Itakura M. A comparison of pretreatment with fentanyl and lidocaine preceded by venous occlusion for reducing pain on injection of propofol: a prospective, randomized, double-blind, placebo-controlled study in adult Japanese surgical patients. Clin Ther 2009; 31: 2107-2112. PMID: 19922881.


20. Fujii Y, Nakayama M. Prevention of pain due to injection of propofol with IV administration of lidocaine 40 mg + metoclopramide 2.5, 5, or 10 mg or saline: a randomized, double-blind study in Japanese adult surgical patients. Clin Ther 2007; 29: 856-861. PMID: 17697904.


21. Han YK, Jeong CW, Lee HG. Pain reduction on injection of microemulsion propofol via combination of remifentanil and lidocaine. Korean J Anesthesiol 2010; 58: 435-439. PMID: 20532050.



22. Scott RP, Saunders DA, Norman J. Propofol: clinical strategies for preventing the pain of injection. Anaesthesia 1988; 43: 492-494. PMID: 3261547.


23. Borazan H, Erdem TB, Kececioglu M, Otelcioglu S. Prevention of pain on injection of propofol: a comparison of lidocaine with different doses of paracetamol. Eur J Anaesthesiol 2010; 27: 253-257. PMID: 19696679.


24. Picard P, Tramèr MR. Prevention of pain on injection with propofol: a quantitative systematic review. Anesth Analg 2000; 90: 963-969. PMID: 10735808.


25. King SY, Davis FM, Wells JE, Murchison DJ, Pryor PJ. Lidocaine for the prevention of pain due to injection of propofol. Anesth Analg 1992; 74: 246-249. PMID: 1731545.


26. Gregory RE, Ettinger DS. 5-HT3 receptor antagonists for the prevention of chemotherapy-induced nausea and vomiting. A comparison of their pharmacology and clinical efficacy. Drugs 1998; 55: 173-189. PMID: 9506240.


27. Kim SI, Kim SC, Baek YH, Ok SY, Kim SH. Comparison of ramosetron with ondansetron for prevention of postoperative nausea and vomiting in patients undergoing gynaecological surgery. Br J Anaesth 2009; 103: 549-553. PMID: 19700442.



28. Ho CL, Su WC, Hsieh RK, Lin ZZ, Chao TY. A randomized, double-blind, parallel, comparative study to evaluate the efficacy and safety of ramosetron plus dexamethasone injection for the prevention of acute chemotherapy-induced nausea and vomiting. Jpn J Clin Oncol 2010; 40: 294-301. PMID: 20026457.



Table 1
Demographic Data
Values are expressed as mean ± SD or number of patients. There were no significant differences among groups. Group N: patients who received normal saline, Group L: patients who received lidocaine 20 mg, Group R: patients who received ramosetron 0.3 mg, Group LR: patients who received ramosetron 0.3 mg plus lidocaine 20 mg.
Table 2
Incidence and Severity of Pain following Microemulsion Propofol Injection
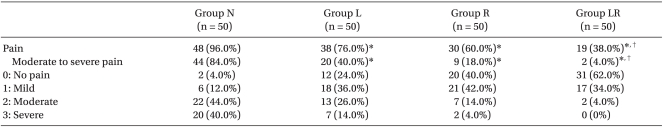
Data are expressed as number of patients (%). Group N: patients who received normal saline, Group L: patients who received lidocaine 20 mg, Group R: patients who received ramosetron 0.3 mg, Group LR: patients who received ramosetron 0.3 mg plus lidocaine 20 mg. *P < 0.008 compared with Group N, †P < 0.008 compared with Group L.
- TOOLS








