Introduction
Remifentanil is a potent, ultrashort-acting opioid with a predictable, rapid recovery. It also results in potentially rapid onset, greater postoperative pain in the immediate postoperative period. As postoperative pain is difficult to relieve once it becomes established, many investigators recommend that the anesthesiologist consider methods to prevent pain before discontinuing the remifentanil infusion when remifentanil is used clinically in patients who are expected to experience postoperative pain [
1,
2].
One such method is to administer a long-lasting opioid before stopping the remifentanil during surgery. However, this might prolong the recovery and reduce the potential advantages of remifentanil [
3]. Continuous remifentanil infusion at a lower analgesic dose postoperatively (0.05-0.23 ┬Ąg/kg/min) is another method [
4]; however, it requires postoperative monitoring and supervision. Few studies have evaluated the effect of continuous remifentanil infusion with other opioidbased IV-patient-controlled analgesia (PCA) immediately after an operation.
We aimed to evaluate and compare the effect and safety of two different doses (0.05 vs. 0.1 ┬Ąg/kg/min) of continuous remifentanil infusion immediately after an operation with alfentanil-based IV-PCA in patients undergoing laparoscopic-assisted vaginal hysterectomy (LAVH).
Materials and Methods
The study protocol was approved by our institutional review board and written informed consent was obtained from all patients before enrolling in the study. Fifty patients who were deemed to be American Society of Anesthesiologists (ASA) physical status 1 or 2, aged at least 20 years old, and scheduled for elective LAVH were enrolled in this study. The exclusion criteria were a history of alcohol or drug abuse, known hypersensitivity to opioids, cardiovascular or psychiatric disease, and chronic exposure to benzodiazepines, tricyclic antidepressants, or anticonvulsants.
All patients were premedicated with midazolam 0.04 mg/kg and glycopyrrolate 0.2 mg intramuscularly 30 minutes preoperatively. When the patient arrived in the operating room, standard monitors were placed, including the electrocardiogram (ECG), pulse oximetry, noninvasive blood pressure cuff, and bispectral index (BIS).
Anesthesia was induced with 4 mg/kg thiopental sodium, 0.7 mg/kg rocuronium, and 0.1 ┬Ąg/kg/min remifentanil. After tracheal intubation, anesthesia was maintained with sevoflurane-O2-air-remifentanil. The sevoflurane concentration and remifentanil infusion were adjusted to maintain a BIS of 40-60 and mean arterial blood pressure (MAP) within 20% of the preoperative value, respectively. Twenty minutes before the anticipated end of surgery, ramosterone 0.15 mg was administered to prevent postoperative nausea and vomiting.
At the last skin suture, the sevoflurane was discontinued, the neuromuscular block was reversed, and the remifentanil infusion dosage was changed to 0.05 ┬Ąg/kg/min (group I) or 0.1 ┬Ąg/kg/min (group II). At the time of eye opening and response to a verbal command, all patients received IV-PCA composed of alfentanil 2 mg, ketorolac 150 mg, ramosterone 0.15 mg, and normal saline in a total volume of 100 ml. The IV-PCA maintenance dose, bolus dose, and lockout interval were 0.03 ml/kg/h, 0.02 ml/kg, and 8 minutes respectively, according to standard practice in our unit. We did not use a loading dose for fear of delayed awakening.
After obtaining adequate respiration, the patients were extubated and transported to the recovery room. In the recovery room, all patients had an oxygen mask to aid their breathing (5-7 L/min). We monitored the blood pressure (BP), heart rate (HR), respiratory rate (RR), SpO2, and BIS at 5-minute intervals. Adverse effects were monitored, including muscle rigidity, shivering, nausea, vomiting, and respiratory depression. Respiratory depression was defined as SpO2 < 90% or RR < 8 breaths/min. When respiratory depression developed, the remifentanil infusion and IV-PCA were discontinued. Thirty minutes after starting IV-PCA, the remifentanil infusion was discontinued. Pain was assessed using a visual analog scale (VAS) (0 = no pain, 100 = the worst possible pain) at 0, 5, 10, and 30 minutes after stopping the remifentanil infusion.
Data are expressed as the mean ┬▒ standard deviation (SD). Statistical analysis was performed using SPSS (version 14.0; SPSS Inc., Chicago, IL, USA). The patient characteristics and the eye opening time in response to a verbal command were compared using Student's t-test. The differences in the MAP, HR, BIS, SpO2, and RR between the two groups were analyzed using repeated-measures analysis of variance (ANOVA). In all tests, P < 0.05 was considered to be significant.
Results
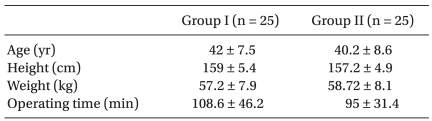
The two groups were comparable in terms of demographic data (
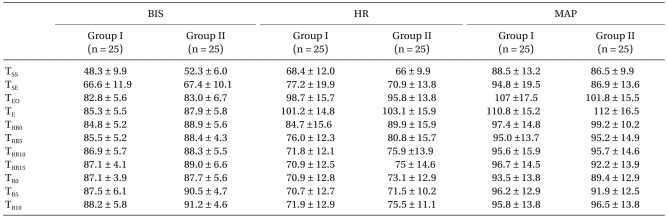
Table 1). The BIS, MAP, and HR did not differ significantly between the two groups (
Table 2). The eye opening times in response to a verbal command were 4.5 ┬▒ 3.2 vs. 6.4 ┬▒ 3.8 min in groups I and II, respectively. The pain scores at 0 minute after stopping the remifentanil infusion were lower in group II, and those 30 minutes after stopping were similar (
Table 3). Side effects, such as muscle rigidity, nausea, or vomiting were not observed. The RR was similar between the groups (
Table 4), although the remifentanil infusion and IV-PCA were stopped in the recovery room in three patients due to respiratory depression in group II. They were fully recovered after stopping the remifentanil infusion and were not administered naloxone.
Discussion
The purpose of this study was to compare the efficacy and safety of continuous remifentanil infusion at two doses (0.05 vs. 0.1 ┬Ąg/kg/min) for providing analgesia in the immediate postoperative period with alfentanil-based IV-PCA, which we have used for pain control after operations.
A constant dose of 0.05 ┬Ąg/kg/min remifentanil with alfentanil-based IV-PCA had an effect similar to 0.1 ┬Ąg/kg/min infusion with respect to pain control without respiratory depression. This result corresponded well with those of earlier studies. Yarmush et al. [
4] found that a 0.05-0.23 ┬Ąg/kg/min remifentanil infusion provides safe, effective postoperative analgesia in the first 35 minutes after extubation. Bowdle et al. [
5] tried to reduce the infusion of remifentanil from an anesthetic dose to an analgesic dose (0.05 ┬Ąg/kg/min) at the conclusion of surgery by titrating the analgesic infusion in the recovery room for 30 minutes followed by a gradual transition to a longer-acting opioid. In that case, morphine was used as a rescue analgesic. In a previous study [
6], a constant 0.1 ┬Ąg/kg/min dose of remifentanil provided adequate analgesia (VAS score, 1.7 ┬▒ 2.0) in 78% of patients without signs of respiratory depression after a 4-hour infusion. That study used meperidine 0.5 mg/kg as a rescue analgesic. They suggested that a 2-4 hour remifentanil infusion in the immediate postoperative period was adequate for ensuring patient adaptation to other analgesics, which proved difficult to initiate within 1 hour. The remifentanil infusion duration and the drug and method for rescue analgesia were variables in each study. We thought that the duration of remifentanil infusion for the analgesic dose in the current study provided enough transition time before discontinuing the remifentanil infusion [
2,
4]. Additional studies are needed to define a transition regimen that will improve postoperative analgesia in patients who received a remifentanil infusion during an operation.
The incidence of respiratory depression is variable [
4,
5,
7]. Bowdle et al. [
5] reported that adverse respiratory events (SpO
2 < 90% or RR < 12) affected 29% of patients and the incidence of apnea was 7%. Sch├╝ttler et al. [
7] reported apnea in 11% and respiratory depression in 10%. In our study, 3 of the 25 patients given the 0.1 ┬Ąg/kg/min remifentanil infusion developed respiratory depression. These patients recovered soon after the remifentanil infusion and IV-PCA was stopped. The causes for the increased incidence of respiratory depression may be the different method of remifenanil infusion rate increments according to VAS, the addition of bolus or not, or the use of other rescue analgesics or not.
Some studies have suggested that remifentanil infusion during surgery results in acute opioid tolerance or hyperalgesia manifested as increased postoperative pain and the need for opioids [
4,
8]. However, acute opioid tolerance and its prevention remain controversial [
9]. Patients treated with remifentanil infusion might suffer from more postoperative pain during the immediate postoperative period [
10]. Postoperative pain is difficult to relieve, especially once it has become established [
5]. A study has suggested that anesthesiologists who use remifentanil as the opioid component of anesthesia must anticipate this rapid onset of pain and provide analgesia appropriate for the degree of anticipated postoperative pain before discontinuing a remifentanil infusion [
4].
Continuing the remifentanil infusion at a lower, analgesic dose immediately after an operation is effective for pain control, but a need to switch to long-acting analgesics still exists before leaving the postanesthetic care unit [
4]. In this study, alfentanil-based IV-PCA was provided after eye opening in response to a verbal command, and a loading dose of PCA or other analgesics was not allowed. Upon stopping the remifentanil infusion, the pain score was 62.4 ┬▒ 30 in group I and 55 ┬▒ 30.2 in group II. These scores were similar to the results of studies that used morphine or meperidine as rescue analgesics and comparable to those of reports that evaluated pain control after LAVH with IV-PCA [
6,
11]. Most patients remained calm and did not complain of pain. We expected a slightly lower pain score, but no significant differences were observed between the two groups. When considering the side effects, such as respiratory depression, the 0.1 ┬Ąg/kg/min remifentanil infusion showed no advantages compared to 0.05 ┬Ąg/kg/min.
A recent clinical study has suggested that remifentanil infusion should not be continued immediately postoperatively, but be given in the form of remifentanil PCA. However, the safety of remifentanil in awake and spontaneously breathing patients during the postoperative period has not been established and the efficacy and safety of remifentanil PCA are controversial. Krishnan et al. [
12] reported that continuing with remifentanil PCA may be considered for patients who receive a remifentanil infusion as a part of anesthesia during surgery. However, special attention must be given to respiratory depression when establishing remifentanil PCA and any remifentanil bolus should be administered only in a controlled fashion and slowly to avoid potential respiratory and cardiovascular side effects. Some studies have reported that remifentanil PCA has no benefits compared to fentanyl or morphine [
13,
14]. Moreover, it is not safe for postoperative analgesia in the general ward, and careful monitoring of respiratory function and skills in the recognition and treatment of inadequate respiration should be obligatory in a clinical setting when using remifentanil [
5,
13]. The high cost of remifentanil might also be a considering factor.
In conclusion, a continuous remifentanil infusion (0.05 ┬Ąg/kg/min) immediately postoperatively with alfentanil-based IV-PCA had a similar effect as a 0.1 ┬Ąg/kg/min infusion with respect to pain control and respiratory depression.