|
 |
| Korean J Anesthesiol > Volume 75(4); 2022 > Article |
|
Abstract
Background
Obstructive fibrinous pseudomembrane tracheitis (OFPT) is a rare complication of endotracheal intubation.
Case
We describe the case of a 73-year-old woman who underwent short-term intubation for video-assisted thoracoscopic surgery and developed an acute life-threatening stridor two days after extubation. The patient required an emergency tracheostomy to maintain airway patency and a microscopic direct laryngoscopy procedure was performed thereafter with removal of the obstructive pseudomembrane. Subsequently, the patient also suffered a non-ST-elevation myocardial infarction. The patient successfully recovered, and the tracheostomy was subsequently decannulated two months later. Histological examination revealed mucosal ulcerations and inflammatory changes.
Endotracheal intubation is widely performed for airway control during anesthesia. Obstructive fibrinous pseudomembrane tracheitis (OFPT) is a rare but life-threatening complication of endotracheal intubation that can mimic other pathologies, such as vocal cord palsy, laryngeal edema, and tracheomalacia. Patients usually present with stridor and voice hoarseness hours to days after intubation. Here, we report the case of a patient who experienced respiratory distress two days after thoracic surgery. Written informed consent was obtained from the patient for publication.
A 73-year-old woman (height: 154 cm, weight: 55 kg) was diagnosed with a malignant lung nodule in the left lower lobe that was incidentally discovered on computed tomography. The patient was a non-smoker with a history of hypertension, non-insulin-dependent diabetes mellitus on oral hypoglycemic agents, and epilepsy on sodium valproate. She had a surgical history of a laparoscopic cholecystectomy 11 years ago, which was uncomplicated according to the patient; however, no records were available for review. Additionally, the patient had a history of hyperthyroidism, had received radioactive iodine treatment more than 10 years prior, and was on oral levothyroxine replacement. The preoperative thyroid function test results were normal, and the patient was clinically euthyroid. No pre-existing voice hoarseness or dyspnea was noted. Her functional capacity was 4–10 metabolic equivalents, as she was able to walk 200 m at normal speed.
On preoperative assessment, the patient had a normal airway (Mallampati grade 2, interincisor distance of at least 5 cm, full neck range of movement, no neck masses), and the patient was edentulous. Routine tests for thoracic surgery showed normal spirometry (FEV1: 1.44 L [85% of the predicted value], FVC: 1.72 L [92% of the predicted value], and DLCO 94% of the predicted value). Transthoracic echocardiography revealed an ejection fraction of 66% and an absence of regional wall motion abnormalities. The patient had aortic valve sclerosis but no stenosis, and all other valves were normal.
The patient underwent video-assisted thoracoscopic surgery for left lower lobectomy. Induction of anesthesia was uneventful, and a direct laryngoscopy was performed, which showed a grade 1 Cormack-Lehane view with no abnormality of the vocal cords. A size 37 left double-lumen endotracheal tube (DLT) was easily inserted on the first attempt, and no excessive pressure or force was used. A DLT of this size was chosen instead of a smaller bore to facilitate fiberoptic bronchoscopy and tracheal suctioning. The bronchial tip was directed into the left mainstem bronchus on the first attempt atraumatically with fiberoptic bronchoscopic guidance. The tracheal cuff was maintained at 20 cmH2O. The DLT was in place for 2 h 10 min, and the surgery was uneventful. Anesthesia was fully reversed, and the patient was then extubated with a face mask. The patient reported mild sore throat, but no hoarseness was noted. She was subsequently discharged from the post-anesthesia care unit.
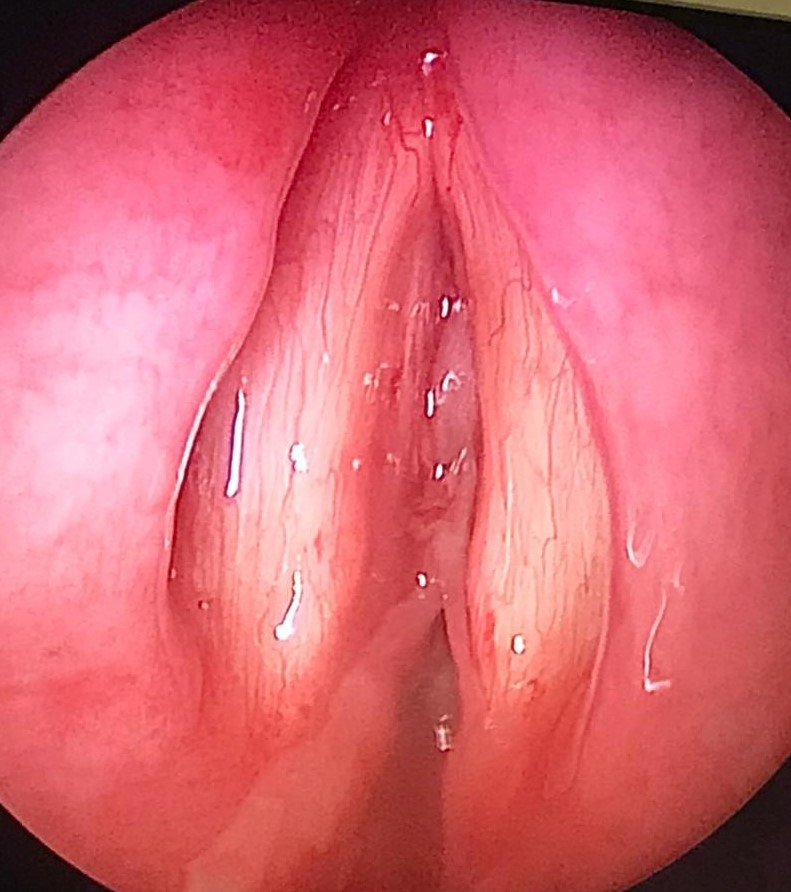
A total of 48 h later, however, she was noted to have a hoarse voice with mild stridor in the ward. No swallowing issues were observed. A bedside nasoendoscopy was performed, which showed anterior and posterior webbing in the subglottic area, just inferior to the level of the true cords, corresponding to Cotton-Myer grade II stenosis (Fig. 1). Since the nasoendoscope was narrow-bored and thus did not have the features to allow for a therapeutic excision to be performed, and because monitoring was limited at the bedside, the membrane was not removed. At this time, the patient only had mild hoarseness and no dyspnea or respiratory distress, and was able to maintain adequate oxygenation on room air. Bronchoscopy was not performed to avoid aggravating the laryngeal edema. She was subsequently started on inhaled budesonide.
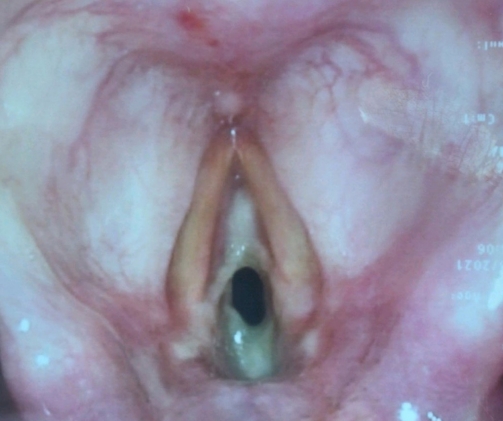
The next day, the patient quickly deteriorated, requiring emergency surgical intervention for critical upper airway obstruction (Fig. 2). The oral pharynx was topicalized with lidocaine, and an awake videolaryngoscopy was performed; however, visualization of the glottic opening was poor, and thus an awake tracheostomy was performed under local anesthesia with oxygenation via a Hudson mask. Upon securing the airway through tracheostomy, a microscopic direct laryngoscopy was performed, which showed white adherent fibrinous material at the glottis and subglottic area, with a residual 1-mm pinpoint glottic gap posteriorly, corresponding to Cotton-Myer grade III subglottic stenosis. The adherent fibrinous material appeared fixed initially, but on palpation, it was found to be removable. After the material was removed using a microlaryngeal forceps, the subglottic area appeared less than 50% stenotic (Cotton-Myer grade I stenosis). The underlying mucosa appeared healthy anteriorly, but the posterior commissure mucosa was edematous. Over the following few days, serial nasoendoscopic evaluation showed that the pseudomembrane had cleared.
The next day, however, the patient suffered a non-ST-elevation myocardial infarction, presenting with T-wave inversion without any angina or evidence of hypoxemia. She underwent percutaneous coronary intervention and stenting of the culprit left anterior descending artery occlusion, which was caused by an underlying structural lesion that had not been detected preoperatively. The patient was subsequently discharged two weeks after hospital admission. Serial outpatient nasoendoscopy showed no reaccumulation of the subglottic pseudomembrane, and the tracheostomy was successfully decannulated two months later.
Histological analysis of the subglottic material revealed inflammatory changes, namely, squamous-lined mucosa with extensive ulceration and formation of organizing fibrinopurulent acute inflammation in the lamina propria. No bacteria or fungi were detected on staining.
OFPT is a rare but potentially fatal complication of endotracheal intubation. However, the exact pathophysiology of pseudomembrane formation remains unclear. One hypothesis is that the subglottic mucosa is prone to injury during intubation because it is the narrowest part of the adult larynx and completely surrounded by the complete ring of the cricoid cartilage. After injury, the accumulation of desquamated epithelial cells may result in the formation of a membranous-like material [1]. Indeed, the most common location of the tracheal pseudomembrane is in the subglottic area, but it can also be found distally, such as in the mid-trachea or carina [2–5].
Risk factors may include female sex, tracheal tube cuff pressure > 25 cmH2O, traumatic intubation, use of a DLT, prolonged endotracheal intubation, airway stenting, a compromised immune system, and bacterial or fungal infection [1,2,6–15]. Additionally, aspiration of gastric contents leading to caustic injury has also been proposed as a contributory factor to the formation of OFPT after an initial disruption of the tracheal mucosa followed by an abnormal healing process [1,13]. Patients should be observed closely, since OFTP can occur in the absence of prolonged intubation, and the time to onset of symptoms after extubation can vary widely [3,5]. Before confirming the diagnosis, patients usually receive empirical treatment consisting of salbutamol bronchodilator, epinephrine, and corticosteroids.
To confirm the diagnosis, an endoscopic evaluation is performed [1,5]. Histology typically reveals the presence of inflammatory infiltrates, fibrin, and ulcerations. Bacteria, viruses, or fungi may also be observed as they may also cause OFPT. Treatment typically involves using rigid or flexible bronchoscopy to remove the pseudomembrane and relieve the obstruction. Rigid bronchoscopy, however, is safer as it can be used to maintain ventilation during the intervention. Although it is uncommon, recurrence of the tracheal membrane can occur and result in repeated obstructions [1].
In conclusion, OFPT is a rare cause of airway obstruction after extubation that is often not immediately recognized, but can usually be treated with mechanical removal of the lesion with bronchoscopic intervention or microscopic direct laryngoscopy as in our patient’s case.
Acknowledgments
The authors would like to thank co-author Brenda Ling Hui Sim for providing the figures.
Fig. 1.
Thick rubbery whitish pseudomembrane at the anterior and posterior region, in the subglottic area, just inferior to the level of the true vocal cords.
References
1. Sehgal IS, Dhooria S, Bal A, Aggarwal AN, Behera D, Agarwal R. Obstructive fibrinous tracheal pseudomembrane after endotracheal intubation. Respir Care 2016; 61: 1260-6.

2. Talwar A, Patel N, Omonuwa K, Lisker G. Postintubation obstructive pseudomembrane. J Bronchology Interv Pulmonol 2008; 15: 110-2.



3. Lins M, Dobbeleir I, Germonpré P, Waelput W, Pauwels P, Jorens PG. Postextubation obstructive pseudomembranes: a case series and review of a rare complication after endotracheal intubation. Lung 2011; 189: 81-6.


4. Vanderheyde K, Pieters T, Rodenstein D. A 19-year-old man with dyspnea and stridor after surgery. Respiration 2011; 81: 63-6.


5. Ammar Y, Vella-Boucaud J, Launois C, Vallerand H, Dury S, Lebargy F, et al. Obstructive fibrinous tracheal pseudomembrane. Anesth Analg 2017; 125: 172-5.


6. Manassero A, Ugues S, Bertolaccini L, Bossolasco M, Terzi A, Coletta G. A very early stage of obstructive fibrinous tracheal pseudo-membrane formation. J Thorac Dis 2012; 4: 320-2.



7. Deslée G, Brichet A, Lebuffe G, Copin MC, Ramon P, Marquette CH. Obstructive fibrinous tracheal pseudomembrane. A potentially fatal complication of tracheal intubation. Am J Respir Crit Care Med 2000; 162: 1169-71.


8. Kawakami N, Ito M, Takahashi K, Moriya T, Saito H, Wakai Y, et al. Pseudomembranous tracheobronchitis with severe tracheal stenosis and masked bronchial obstruction. J Emerg Med 2021; 60: e39-e44.


9. Lee YH, Seo H, Cha SI, Kim CH, Lee J. A case of pseudomembranous tracheitis caused by Mycoplasma pneumoniae in an immunocompetent patient. Ann Transl Med 2019; 7: 205.



10. Grosu HB, Bashoura L, Ost D, Ordonez NG, Faiz SA. Critical airway obstruction due to pseudomembranous Aspergillus tracheitis. Am J Respir Crit Care Med 2014; 190: e65-6.


11. Sahu SR, Madan K, Mohan A, Mittal S. Obstructive fibrinous tracheal pseudomembrane following tracheal stent placement: an underrecognized entity. Lung India 2020; 37: 453-4.



12. Yildirim BB, Karalezli A, Hasanoglu HC, Kandemir O. Obstructive fibrinous tracheal pseudomembrane. J Bronchology Interv Pulmonol 2012; 19: 129-31.


13. Kang HH, Kim JW, Kang JY, Kim JS, Kim MS, Kim SS, et al. Obstructive fibrinous tracheal pseudomembrane after tracheal intubation: a case report. J Korean Med Sci 2010; 25: 1384-6.