|
 |
| Korean J Anesthesiol > Volume 74(4); 2021 > Article |
|
Cold agglutinin disease (CAD) is an autoimmune disease caused by higher titers of cold-reacting autoantibodies that lead to red blood cell (RBC) agglutination and subsequent hemolysis at low temperatures [1]. In such patients, exposure to cold can lead to hemoglobinuria and critical complications such as renal failure and myocardial damage [1]. Therefore, the prevention of perioperative hypothermia is crucial in these patients undergoing surgery. Herein, we report the successful perioperative management of a patient with severe CAD by using multimodal warming measures, as described below. Written informed consent was obtained from the patient for publication of following case.
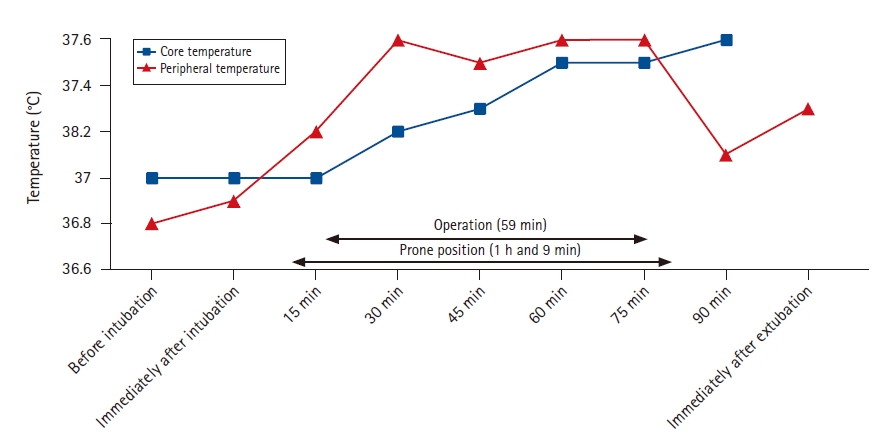
A 63-year-old man diagnosed with CAD for 2 years based on repeated clinical symptoms with high titers of cold agglutinin (1:8192) measured at ambient temperature, was scheduled to undergo lumbar laminectomy for lumbar canal stenosis. Despite being on daily medication with prednisolone 10 mg, he often experienced hemoglobinuria and acrocyanosis of the distal extremities upon exposure to cold even in summer. On the day of the surgery, to prevent perioperative hypothermia, the infusion of amino acid warmed at 41°C with HOTLINE® Warmer (Smiths Medical Japan Ltd., Japan) was commenced 3 h before surgery. Skin-surface warming with an electric heating blanket was also commenced 30 min before surgery, and the operating room was pre-warmed to 26°C. In the operating room, after anesthesia induction, a temperature probe was inserted into the esophagus immediately before orotracheal intubation. Following intubation, the patient was placed in a prone position, and forced-air warming devices (EQUATOR®; Smiths Medical Japan Ltd., Japan) set at 37°C to 40°C were immediately applied to his upper and lower body. His anterior skin surfaces were also warmed using a circulating-water mattress, and his hands and feet were covered with gloves and socks, respectively. Immediately before skin incision, 100 mg hydrocortisone was administered intravenously for steroid cover. During surgery, intravenous infusion of amino acid warmed at 41°C was also continued. Surgical irrigation solutions were also pre-warmed to approximately 40°C. Fig. 1 shows the intraoperative trends of the core (esophagus) and peripheral (palm) temperatures. The patient was warmed continuously with an electric heating blanket and warmed amino acid infusions for approximately 24 h after surgery. The postoperative course was uneventful, without any evidence of hemolysis.
In patients with CAD, when the blood cools below a critical temperature, often ranging from 30┬░C to 37┬░C, which is called the thermal threshold, cold sensitive antibodies cause RBC agglutination and complement fixation. Thus, it is extremely crucial to maintain the core temperature and prevent further heat transfer from the core to the peripheries in these patients. Considering that the patientŌĆÖs normal temperature was approximately 36.5┬║C in the present case, to ensure the safety margin, we aimed to maintain both the core and peripheral temperatures at approximately 37.0┬░C using multimodal warming measures such as preoperative warming [2], intraoperative forced-air warming [1], administration of pre-warmed intravenous fluids [3], and infusion of amino acids [3], which have been reported to effectively prevent perioperative hypothermia. For example, Young and Haldane [4] described the efficacy of intraoperative forced-air warming and administration of pre-warmed intravenous fluids in the management of a patient with CAD. In the present case, in addition to the conventional strategies used [4], we applied two more effective measures, including preoperative skin-surface warming [2] and perioperative warmed amino acid infusion [3], to ensure a safety margin because the patientŌĆÖs CAD was clinically severe. As shown in Fig. 1, while pre-warmed amino acid infusions mainly contributed to the maintenance of the core temperature, skin-surface warming helped to maintain both the core and peripheral temperatures by avoiding heat transfer from the core to the peripheries because of increased peripheral temperatures. Intraoperative amino acid infusions enhance thermogenic effects and prevent hypothermia effectively during general anesthesia [5]. However, amino acid infusions started after the development of core hypothermia failed to accelerate rewarming [5]. Accordingly, for the prevention of hypothermia, amino acids should be administered before the development of core hypothermia. That is, the prophylactic administration of amino acids should be considered in severe cases where core hypothermia must be avoided. In the present case, the infusions of the amino acid were pre-warmed at 41┬░C. A previous report [3] described that the administration of amino acids heated to 40ŌĆō42┬░C safely reduced the incidence of intraoperative hypothermia. Therefore, we considered that the administration of amino acids warmed to 41┬░C could safely and effectively prevent intraoperative hypothermia in the present case. We also used surgical irrigation solutions pre-warmed to approximately 40┬║C because these solutions warmed to 39┬║C were safely used in preventing intraoperative hypothermia in a previous report [1].
To our knowledge, this is the first report describing the efficacy of the combination of these four strategies for the prevention of perioperative hypothermia in patients with severe CAD. The most striking difference between the present case and previous reports [1ŌĆō3] is that warmed amino acid infusion and other preventive measures were simultaneously applied in our severe case. We were able to successfully maintain the core temperature and avoid further heat transfer from the core to the peripheries perioperatively, using these multimodal warming measures. In conclusion, in the management of patients with severe CAD, multimodal warming measures from various viewpoints should be considered to maintain the core temperature and avoid initial thermal redistribution from the core to the peripheries by reducing the temperature gradient between the two regions.
References
1. Cao X, White PF, Ma H. Perioperative management of a patient with cold agglutinin disease undergoing segmental hepatectomy. Minerva Anestesiol 2017; 83: 340.


2. Horn EP, Bein B, B├Čhm R, Steinfath M, Sahili N, H├Čcker J. The effect of short time periods of pre-operative warming in the prevention of peri-operative hypothermia. Anaesthesia 2012; 67: 612-7.


3. Jin L, Han X, Yu Y, Xu L, Wang H, Guo K. Intraoperative thermal insulation in off-pump coronary artery bypass grafting surgery: a prospective, double blind, randomized controlled, single-center study. Ann Transl Med 2020; 8: 1220.