Introduction
Central venous catheterization utilizing anatomical landmarks via the right internal jugular vein (RIJV) is successful in most cases, and it offers the advantage of a linear course directed towards the right atrium [
1]. However, this commonly performed maneuver is not without complications, most of which are associated with direct trauma from needling. The most common complication is accidental carotid artery puncture (reported incidence, 1.9-15%) [
1,
2], which can cause airway obstruction due to hematoma formation [
3]. Secondary complications include thromboembolism and arteriovenous fistula formation [
4,
5,
6]. The incidence of such complications is directly related to the number of needling attempts during central venous catheterization [
7,
8].
Having a large internal jugular vein (IJV) can increase the success rate of first pass attempts [
9]; thus, several investigators have proposed methods to engorge the vein. Hollenbeck et al. [
10] achieved an increase in RIJV cross-sectional area (CSA) by applying a positive-end expiratory pressure (PEEP) of 10 cmH
2O in patients under general anesthesia, while Marcus et al. [
11] studied the degree of increase in RIJV CSA following the application of 5 and 10 cmH
2O PEEP, with or without 20° of Trendelendburg tilting. These studies measured the RIJV CSA at its largest, without considering changes in CSA according to the respiratory cycle. The IJV CSA is affected by respiratory fluctuations, and, theoretically, these differences are greater in patients under positive pressure ventilation. As the majority of central venous catheterizations in perioperative settings are performed on patients under positive pressure ventilation [
12,
13], it is worth investigating the effects of respiratory fluctuations on the IJV CSA.
The purpose of this study was to investigate changes in the size (e.g., CSA, anteroposterior [AP] diameter, and transverse diameter) of the RIJV with respect to the respiratory cycle in patients under general anesthesia and positive pressure ventilation. We also examined the effects of PEEP and the Trendelenburg position on the size of the RIJV during the respiratory cycle.
Materials and Methods
This prospective, single blind and experimental study was approved by the Institutional Review Board. Written informed consent was obtained from all subjects. We enrolled 24 American Society of Anesthesiologists physical status I-II patients undergoing elective gynecologic or orthopedic surgery under general endotracheal anesthesia. The exclusion criteria were a history of neck surgery, previous RIJV cannulation, cardiac disease, and pulmonary disease. Patients were excluded after enrollment if severe hypotension after the induction of anesthesia occurred.
No other opioid or sedative premedication was administered. Routine monitoring of the subjects' blood pressure, an electrocardiogram, and pulse oximetry were performed. Anesthesia was induced with propofol and remifentanil using a target-controlled infusion system (Orchestra® Base Primea; Fresenius Vial, Brezins, France). Neuromuscular blockade was performed with rocuronium (0.6 mg/kg). Following tracheal intubation, patients were placed on mechanically controlled ventilation (volume-controlled mode). Ventilation was set at 6-8 ml/kg for the tidal volume, a respiratory rate of 10 breaths per minute at an inspiration-to-expiration time ratio of 1 : 2, and an end tidal carbon dioxide level of 30-40 mmHg. The ventilator settings were fixed while performing the study.
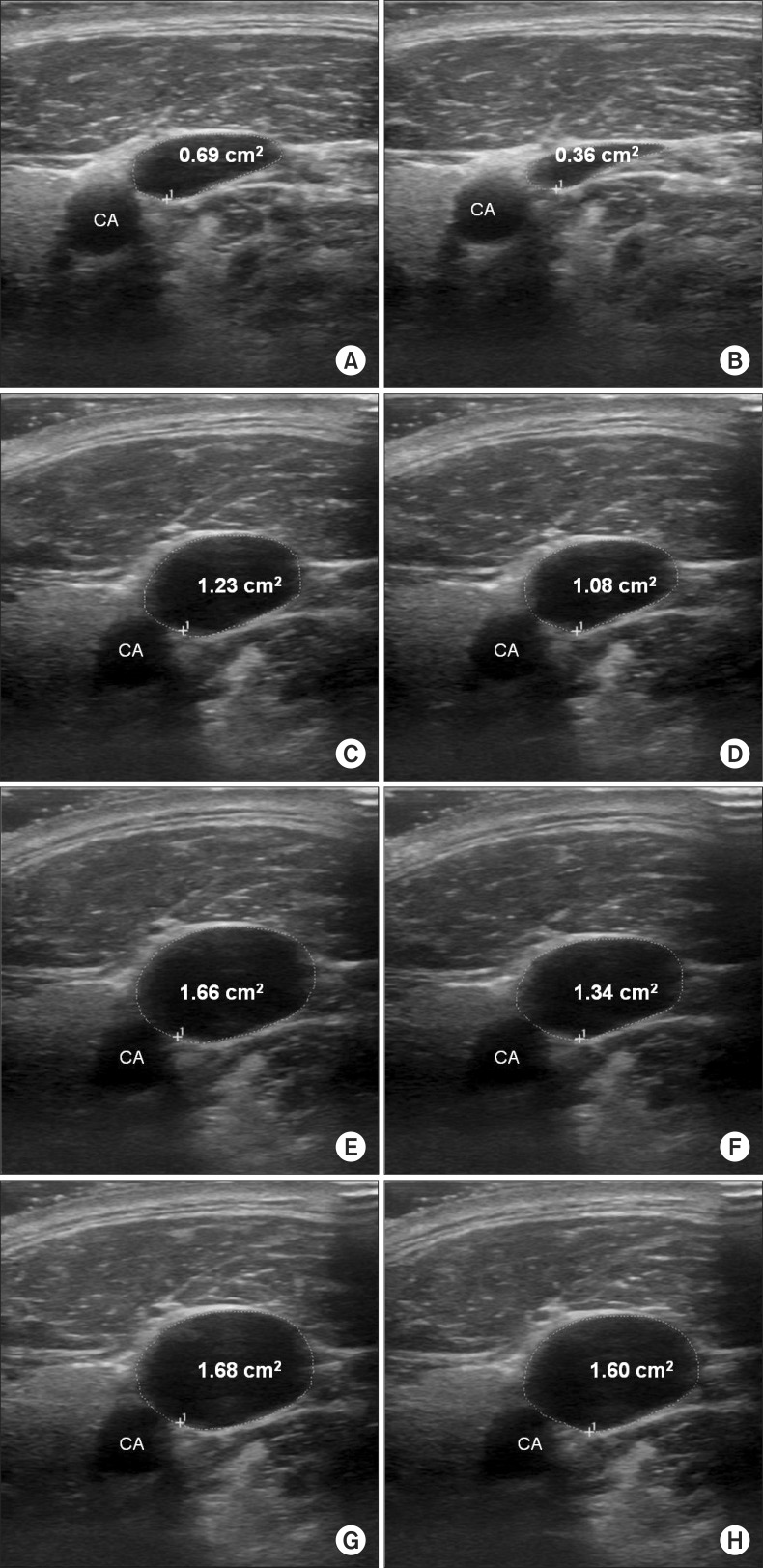
With the patient in the supine position, PEEP = 0 (S0), and no head rotation, a high-frequency linear array transducer (LOGIQe; GE Medical, Jiangsu, China) was used to acquire a sufficient sonographic image of the RIJV by placing the probe perpendicularly over the skin at the cricoid cartilage level (longitudinal plane) with minimal pressure applied. Depending on the ventilation, the RIJV varied in size; the image was frozen and stored when the largest and smallest areas were shown. Whilst holding the probe in the same position, the RIJV was imaged following the application of three different maneuvers in random order: (1) a PEEP of 10 cmH
2O (S10), (2) a 10° Trendelenburg position (T0), and (3) a PEEP of 10 cmH
2O and a 10° Trendelenburg position (T10). Images of the RIJV were obtained at least 30 s after each maneuver, and in between the maneuvers, a minimum of 30 s interval was given at S0 (
Fig. 1). The operating table was tilted 10° to a Trendelenburg position using the Clinometer HD (plaincode™, Schloβberg, Germany) Smartphone application.
The circumference of the RIJV was measured using an electronic marker, and an ultrasound unit was used to calculate the CSA. The examiner was blinded during the procedure. Using the ultrasound unit's embedded measuring system, the transverse (lateral to medial border of the wall) and AP (inferior to superior border of the wall) diameters of the vein were measured.
The patients' hemodynamics were monitored throughout the study. A 30% decrease in systolic blood pressure below the baseline value was defined as hypotension; upon occurrence, patients were treated immediately with 5 mg of ephedrine. A heart rate below 45 beats per minute was promptly raised by administering 0.5 mg of atropine. The use of vasoactive drugs was recorded along with the patients' systolic blood pressure and heart rate. All subjects fasted for 8 h prior to the operation and received lactated Ringer's solution over the first hour (4 ml/kg).
The primary outcome measure was the difference in CSA of the RIJV at the largest and smallest measurements during S0, S10, T0, and T10. The secondary outcome measures were the differences between the transverse and AP diameters at the largest and smallest points during S0, S10, T0, and T10. We also examined the systolic blood pressure and heart rate of the subjects in each position.
Statistics
Randomization was achieved using the Orthoganol Latin Square Design method [
14]. In each of the six sequences in the Orthogonal Latin Square Design, four patients were randomly assigned. One of the six sequences was randomly selected. Upon initiation of the study protocol, both PEEP and the Trendelenburg position were performed according to the order of the randomly assigned sequence.
SAS version 9.1 (SAS Institute, Cary, NC, USA) was used for all analyses. The statistical significance of the respiratory change in the RIJV CSA, and the transverse and AP diameters were analyzed using Generalized Estimating Equation analysis. The P values obtained using these models were adjusted using the Hochberg method. The statistical significance of the changes (largest and smallest, respectively) in the CSA of the RIJV, and the transverse and AP diameters following the application of the different maneuvers was analyzed using a mixed model. The P values obtained using this model were adjusted using Scheffé's method. P < 0.05 was considered statistically significant.
The largest and smallest CSAs at S0 and S10 from ten patients were collected; the mean and standard deviation of the variation in CSA was used to determine the sample size. In a pilot study of ten subjects, the average changes in S0 and S10 were 0.34 ± 0.18 and 0.16 ± 0.11 (mean ± SD), respectively. A minimum sample size of 22 was calculated using α = 0.05, a power of 90%, and 20% dropout. Thus, a sample size of 24 patients was required to incorporate the Prescott triple Latin square design [
14].
Results
The patient characteristics are shown in
Table 1. Seven of the patients were male (29%) and 17 were female (71%). Most of the females were gynecology patients. Complete data and images suitable for analysis were obtained for all patients. No complications were encountered.
Table 2 shows the changes in CSA, transverse diameter, and AP diameter for the largest and smallest RIJV according to the position or PEEP application and the difference between inspiration and expiration. The combination of the two maneuvers (T10) produced a 4.4% decrease in the CSA respiratory change in the RIJV from a 28.3% decrease at baseline (S0) (P = 0.0004; GEE analysis). The effect of PEEP and the Trendelenburg position alone on the respiratory change-induced decrease in CSA of the RIJV was 8.5 and 8.0%, respectively (all P = 0.0004; GEE analysis). In all patients, the CSA, transverse diameter, and AP diameter of the largest and smallest RIJV increased with PEEP application, Trendelenburg position, and both treatments combined.
The systolic blood pressure decreased significantly by 7.0 mmHg, following the application of 10 cmH
2O PEEP (P = 0.024; GEE analysis) (
Table 3). In a 10° Trendelenburg position (T0) and a PEEP of 10 cmH
2O and a 10° Trendelenburg position (T10), the heart rate decreased significantly with the Trendelenburg position (P = 0.0005 and P = 0.004, respectively; GEE analysis) (
Table 3). In addition, no requirement for ephedrine or atropine due to hypotension or bradycardia occurred during the study period.
Discussion
This study was designed to evaluate the effect of the respiratory cycle on the size of the RIJV and to determine the impact of PEEP and the Trendelenburg position in anesthetized adult patients under positive pressure ventilation. During positive pressure ventilation, the CSA was its largest at inspiration and smallest at expiration, with a mean decrease in size from largest to smallest of 28.3 ± 14.7%. Furthermore, with either the Trendelenburg position or PEEP application, this difference was reduced. When combined, the difference decreased further; the largest and smallest CSAs were 2.25 ± 0.76 and 2.14 ± 0.69 cm2, respectively.
Ultrasound-guided RIJV puncture compared to regular RIJV puncture resulted in a lower number of puncture attempts, a higher success rate, and an increased chance of a successful puncture on the first attempt, thus reducing complications [
15,
16,
17]. Nonetheless, ultrasound-guided IJV cannulation caused certain complications, the most frequent being carotid artery puncture at a rate of 1.4% [
18]. To reduce complications, numerous studies have sought to create a method for increasing vein size [
19,
20,
21]. Placing the patient at Trendelenburg positions and using the valsalva maneuver, inspiratory hold, and PEEP can increase the size of the IJV [
10,
11,
12,
15,
19,
20,
21,
22]. However, previous studies focused only on maximizing the vein size, without considering changes in size according to the respiratory cycle.
To minimize complications such as carotid artery puncture, it is recommended to maximize the IJV diameter by controlled ventilation at end-inspiration which reduces the collapsibility of the vein during puncture; thus, the maneuver ultimately reduces any unnecessary additional advancement of the needle during central venous catheterization procedure, lowering the chance of artery puncture [
13]. Because attempting insertion of the needle during spontaneous ventilation by synchronized timing according to respiratory cycle would be rather difficult, the authors suggest to insert the needle after induction rather than before, timing puncture at end-inspiration after intermittent positive ventilation [
13]. Our study showed that both the Trendelenburg position and PEEP application not only increased the size of the vessel, but also reduced fluctuations in size caused by the respiratory cycle. The two in combination resulted in a more effective decrease in fluctuations according to the respiratory cycle; hence, a synchronized puncture according to the respiratory cycle would not be necessary.
Our study used several parameters to determine the size of the IJV: CSA, AP diameter, and transverse diameter. Previous studies have described increases in diameter, but the vein was ovoid rather than round [
20,
21]. Thus, the term diameter is vague and cannot distinguish between the AP and transverse diameters. Our study shows that use of the Trendelenburg position or PEEP application alone minimally changed the transverse diameter while increasing the AP diameter to increase the CSA. This increase reduced the possibility of transfixation caused by needle pressure while puncturing the vein [
12,
23], leading to a reduced likelihood of posterior wall puncture and a reduced chance of carotid artery puncture [
9]. If the transverse diameter had been used in previous studies, the results could have been underestimated. Therefore, the study's primary outcome was set to the change in CSA, and to determine whether the change in CSA was due to the transverse diameter or AP diameter. Changes in the transverse diameter and AP diameter were set as secondary outcomes. Our results indicate that the Trendelenburg position and/or PEEP application increased the CSA, mainly by increasing the AP diameter.
Clenaghan et al. [
21] studied the change in RIJV diameter in healthy subjects in different Trendelenburg positions (10, 15, 20, 25, and 30°). The authors reported that even a 10° tilt is effective while a 25° Trendelenburg tilt achieved the optimum distension. The authors recommended using a 10° tilt. Lobato et al. [
12] found that a 10° Trendelenburg position increased the RIJV CSA by 25% and further increase combining hepatic compression and/or inspiratory hold while a 20° Trendelenburg position resulted in no further increase. Moreover, the degree of PEEP used in the current study was based on previous studies, which showed that PEEPs of a PEEP of 10 cmH
2O increased the CSA of the right internal jugular vein by 22.3 and 41.0%, respectively [
10,
11]. Accordingly, we chose a 10° Trendelenburg tilt and a PEEP of 10 cmH
2O.
Two major limitations are evident in this study. First, changes in IJV CSA are directly related to successful cannulation. The hypothesis that a larger target vessel size would increase the success rate of line placement was supported, but not proven, by this study. Second, the observers were not blinded to the maneuvers. However, the order in which the three maneuvers were applied was randomized, and a blinded examiner measured the CSA and transverse and AP diameters using stored images.
A 10° Trendelenburg tilt position combined with a PEEP of 10 cmH2O reduced respiratory fluctuations in the RIJV; specifically, it decreased the mean difference in CSA, AP diameter, and transverse diameter during the respiratory cycle by 4.4, 2.4, and 1.9%, respectively, compared with the baseline (28.3, 20.8, and 10.3%). Moreover, the combination of the two caused no decrease in systolic blood pressure from baseline. To minimize fluctuations in RIJV size according to the respiratory cycle, and to maximally increase its size, we recommend a 10° Trendelenburg tilt position combined with a PEEP of 10 cmH2O during needle insertion for cannulation of the RIJV under positive pressure ventilation.